Abstract
Eukaryotic algae and cyanobacteria produce hydrogen under anaerobic and limited aerobic conditions. Here we show that novel microalgal strains (Chlorella vulgaris YSL01 and YSL16) upregulate the expression of the hydrogenase gene (HYDA) and simultaneously produce hydrogen through photosynthesis, using CO2 as the sole source of carbon under aerobic conditions with continuous illumination. We employ dissolved oxygen regimes that represent natural aquatic conditions for microalgae. The experimental expression of HYDA and the specific activity of hydrogenase demonstrate that C. vulgaris YSL01 and YSL16 enzymatically produce hydrogen, even under atmospheric conditions, which was previously considered infeasible. Photoautotrophic H2 production has important implications for assessing ecological and algae-based photolysis.
Similar content being viewed by others
Introduction
Microalgae are microorganisms that use solar energy to combine water with carbon dioxide to create biomass1,2. The growth kinetics of photoautotrophic algae depend on the extent of available carbon sources (for example, bicarbonate), which are mainly generated from the dissolution of atmospheric carbon dioxide3,4,5,6. The common photosynthetic process in algae results in the splitting of H2O and the resultant O2 evolution (driven by light absorbed by photosystem II). Subsequently, electrons are transferred from photosystem I reaction centres to hydrogenase (H2ase) through ferredoxin, which is the natural electron donor (driven by light absorbed by photosystem I), while forming biomass or producing energy7,8,9. Under special conditions (anaerobic conditions plus light), photosynthetic electrons are delivered to [FeFe]-H2ase instead of NADPH via ferredoxin, and [FeFe]-H2ase utilizes them to reduce protons to molecular hydrogen. This reaction is catalysed by [FeFe]-H2ase, which is extremely sensitive to oxygen and does not require any extra energy in the form of ATP10. In the meantime, cyanobacteria may produce hydrogen either as a by-product of nitrogen fixation using nitrogenase11 or by a reversible NAD(P)H-dependent NiFe-H2ase12.
Certain unicellular green algae have been well established to be capable of hydrogen metabolism, as has been observed in many taxonomically diverse species, through a variety of biochemical mechanisms and processes with physiological adaptations13,14. Green microalgae, such as Chlamydomonas reinhardtii, C. noctigama, C. moewusii and Chlorella fusca, synthesize [FeFe]-H2ase that is highly active in hydrogen evolution15. However, owing to the oxygen sensitivity of [FeFe]-H2ases, most studies on photosynthetic hydrogen production have largely relied on experimental manipulations, including intermittent illumination and/or anaerobic conditions2,16 (for example, dissolved oxygen was completely removed from the culture media). A review of previous studies revealed that there are no reports on the occurrence of photoautotrophic H2 production in the presence of CO2 and O2.
Here we show that eukaryotic green algal strains can produce H2 during inorganic carbon uptake under high O2 levels that are known to inhibit H2ase synthesis and activity. Two eukaryotic microalgal strains, C. vulgaris YSL01 and YSL16, directly produce gaseous hydrogen through photosynthesis under different dissolved oxygen regimes and continuous illumination, using CO2 as the sole carbon source. The identification of eukaryotic and oxygenic photoautotrophs that can produce hydrogen even under aerobic conditions has important implications for biohydrogen production.
Results
Microalgae can produce hydrogen under aerobic conditions
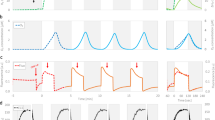
Photoautotrophic hydrogen production by C. vulgaris YSL01 increased from 0.3 to 0.6 ml l−1 in 3 days as headspace CO2 increased from 5 to 15% at 15% O2. C. vulgaris YSL16 produced 0.1 ml l−1 of photoautotrophic hydrogen in 4 days under 5% CO2 and 15% O2 conditions, and increasing CO2 to 15% with 15% O2 improved the hydrogen production to 0.3 ml l−1 in 5 days (Fig. 1a and Supplementary Fig. 1). Under 5% O2 and 10% CO2, C. vulgaris YSL01 and YSL16 produced 1.9 and 1.2 ml l−1 hydrogen in 3 and 4 days, respectively, and increasing the O2 concentration to 15% with 10% CO2 decreased hydrogen production to 1.2 and 0.6 ml l−1 for 3 and 4 days of cultivation, respectively (Fig. 1b and Supplementary Fig. 1). The biomass-normalized hydrogen production (ml of H2 per g of biomass) was similar for elevating headspace CO2 (Fig. 1c), but it significantly decreased from 1.8±0.01 and 1.1±0.03 to 0.6±0.02 and 0.3±0.01 ml g−1 (P<0.0001), with the elevation of O2 levels from 5 to 15% at 10% CO2 for YSL01 and YSL16, respectively (Fig. 1d). We used the one way ANOVA test within the GraphPad Prism5 software to generate all P-values. Hydrogen production was even observed in the control atmospheric CO2 and O2 condition. Cumulative hydrogen production was relatively higher for C. vulgaris YSL01 compared with YSL16 (Fig. 1). The produced and accumulated hydrogen decreased in response to increasing oxygen content in the headspace, and it was completely removed within 7 days after hydrogen production peaked (Fig. 2 and Supplementary Fig. 1). No further hydrogen production was observed at very high partial pressures of oxygen for both C. vulgaris YSL01 and YSL16. An analogous observation was previously reported, in which hydrogen was consumed by photoheterotrophic eukaryotes under the dark/light anaerobic conditions2,4.
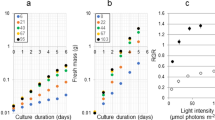
Hydrogen produced by algae accumulated in the headspace of serum bottles under various cultivation conditions. (a,b) The amount of hydrogen in the headspace was monitored throughout the cultivation period, and the highest net hydrogen peak (observed at 3, 4 or 5 days of cultivation) for each culture is shown. The highest level of hydrogen production was achieved by C. vulgaris YSL01 (up to 1.9 ml of H2 l−1 with 5% O2 at 10% CO2). (c,d) Biomass-normalized hydrogen production was similar for increasing CO2 concentrations from 5 to 15% at 15% O2, whereas it significantly decreased with increasing O2 concentrations from 5 to 15% at 10% CO2. (e) The initial biomass concentration of C. vulgaris YSL01 was 0.6 g dry weight l−1, and this value varied from 0.6 to 1.7 g dry weight l−1 after 8 days of cultivation at different initial CO2 concentrations ranging from 0.003 (atmospheric) to 15%. (f) C. vulgaris YSL01 had a similar growth rate, with different initial O2 levels ranging from 5 to 15% at 10% CO2. For all of the cultivation conditions, the suspension pH in the culture bottles increased from 7.9 (initial) up to 8.7 (end of incubation). Each data point represents the average of triplicate experiments and the ±s.d.
Quantification of the H2ase mRNA synthesized by (a–d) C. vulgaris YSL01 and (e–h) C. vulgaris YSL16 during photoautotrophic cultivation under atmospheric and anaerobic conditions. The kinetics of hydrogen production by two different microalgal isolates was monitored for up to 34 days under (a,e) atmospheric and CO2-limited (closed system) as well as (c,g) anaerobic, 10% CO2, and photoautotrophic conditions using crimp-sealed culture bottles. The highest hydrogen content achieved by C. vulgaris YSL01 and YSL16 under atmospheric condition was 0.17 and 0.23%, whereas under the anaerobic condition at 10% CO2, maximum hydrogen contents of 24.6 and 18.2% were achieved, respectively. (b,f) During the period of aerobic hydrogen production, both normalized HYDA expression level and in vitro H2ase activity were quantitatively determined. HYDA was expressed at various concentrations of oxygen, and its relative mRNA concentration displayed a linear correlation with H2ase activity. In vitro H2ase activity was significantly decreased as the oxygen levels increased (≥21%; P<0.05). (d,h) Under anaerobic conditions, H2ase activity and mRNA expression peaked after 4–6 days of cultivation and then dropped significantly owing to the gradual accumulation of oxygen. The value at each time point represents the average of three independent experiments±s.e. The size of the error bars is smaller than the symbol size.
Increasing the headspace CO2 improved the biomass production from 1.2 to 1.7 g l−1, whereas increasing the O2 level had no profound effect on biomass production (Fig. 1e,f). Continuous illumination for the cultivation of these microalgal species resulted in relatively faster growth rates compared with previous studies employing dark/light cycles17,18. The microalgal growth under continuous illumination was mainly dependent on CO2, with little dependence on O2. C. vulgaris YSL01 and YSL16 showed the maximum growth rate of 0.11 and 0.08 per day at 15% CO2 under the investigated experimental conditions (Fig. 1 and Supplementary Fig. 2).
Hydrogen production at high oxygen concentration
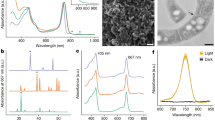
The H2ase gene (HYDA) was expressed and detected even at high concentrations of oxygen (≥21%, initially atmospheric condition), and its relative concentration showed a linear correlation (R2=0.94 and 0.63 for YSL01 and YSL16, respectively) with photosynthetic hydrogen production under the given experimental conditions (Fig. 2a,c). In vitro H2ase activity also supported the changes in hydrogen production (Fig. 2b,d and Supplementary Fig. 3). These results are indicative of the oxygen tolerance of H2ase in the algal cells exposed to high oxygen concentrations for a long period of time. The headspace hydrogen reached a maximum concentration of 0.15–0.69% within 7 days of cultivation for both algal species (Supplementary Fig. 1). Under anaerobic conditions, higher H2ase activity and messenger RNA expression resulted in significantly more hydrogen production compared with the atmospheric environment. The H2ase activity of YSL01 reached a maximum (43.86±1.68 U mg−1 of protein; P<0.05) after 5 days of cultivation, which correlated with the mRNA expression and hydrogen production. An increase in the headspace O2 concentration (>10%) due to microalgal photosynthetic activity decreased the H2ase activity and hydrogen production (Fig. 2c,g).
Figure 3 shows the microalgal hydrogen production and consumption under CO2-enriched aerobic conditions in response to the variations in the headspace oxygen levels, which varied from 15 to 26% as a result of photosynthetic O2 production. Hydrogen production ceased and the subsequent consumption of the accumulated hydrogen occurred when the oxygen level in the headspace exceeded the critical O2 concentration (that is, >21% O2). The headspace was flushed at intervals with a gas mixture containing 15% O2, 15% CO2 and 70% N2, and a similar trend was observed regarding the fate of hydrogen in the headspace. To ascertain whether the H2ase was reactivated or resynthesized, we quantified H2ase activity and mRNA expression at 10 days when hydrogen production had ceased. The H2ase activity and mRNA expression decreased to a value similar to those of day 0 (Fig. 2), suggesting that the hydrogen production observed after flushing the headspace with the gas mixture might be because of the reactivation of H2ase, which was inactivated above the critical concentration of oxygen (Fig. 3). The potential reactivation of this enzyme was demonstrated by determining the specific H2ase activity in the algal cells grown under anaerobic conditions, followed by their subsequent exposure to aerobic and anaerobic environments. The exposure to aerobic conditions inhibited H2ase activity by 72.5%, but the subsequent exposure to anaerobic conditions reactivated the H2ase, restoring 73% of the initial specific H2ase activity (Supplementary Table 1). Notably, H2ase was reactivated even after exposure to 25% O2 for more than 48 h (Fig. 3).
The headspace was recirculated at intervals with a gas mixture composed of 15% O2, 15% CO2 and 70% N2, and photoautotrophic hydrogen production was monitored throughout the 18-day cultivation period. Hydrogen production ceased and the sequential consumption of the accumulated hydrogen occurred when the oxygen level in the headspace exceeded the critical oxygen concentration. Individual data points represent the average of two independent experiments.
H2ase activity correlates with initial oxygen levels
In vitro H2ase activity in both microalgal strains cultivated under various headspace oxygen levels was determined after replenishment with oxygen-free nitrogen for 5 min to activate the H2ase under strictly anaerobic conditions. A decrease in the specific H2ase activity and in vitro H2ase activity was observed for both microalgal species with increasing initial headspace O2, indicating that H2ase activity was strongly influenced by the amount of oxygen (Fig. 4 and Supplementary Fig. 3). These data support the significant decrease in hydrogen production with higher initial O2 concentration (Fig. 1b,d). Relatively higher H2ase activity was observed under lower initial O2 conditions (5–10%) compared with the higher O2 environment (atmospheric O2), but no apparent correlation was observed between the specific H2ase activity and the microalgal biomass amount as the activity significantly varied for similar biomass when the inorganic carbon concentration was fixed at 10% (Figs 1f and 4). H2ase is extremely sensitive to oxygen, and its activity is inhibited even on exposure to low concentrations of oxygen19. However, in the present study, H2ase activity at partial pressure of oxygen (5–21%) consistently indicated that the microalgae could perform hydrogen production and/or H2ase synthesis, even in 21% oxygen (Figs 2 and 4 and Supplementary Fig. 3).
The specific activity of H2ase (empty symbols) in (a) C. vulgaris YSL01 and (b) C. vulgaris YSL16 cultured under different O2 levels in the headspace at 3, 5 and 6 days of cultivation was determined after flushing the microalgal biomass (filled symbols) with oxygen-free nitrogen gas for 5 min to activate H2ase under strictly anaerobic conditions. The specific activity of H2ase decreased with increasing initial O2 in the headspace for the investigated microalgal species, indicating that the enzyme activity was strongly influenced by the amount of oxygen. The numbers in parentheses represent the initial CO2 concentrations. Individual data points represent the average of two independent experiments.
Discussion
This study demonstrates that two novel microalgal strains (C. vulgaris YSL01 and YSL16) are capable of photoautotrophic hydrogen production during exposure to continuous illumination under aerobic conditions. Photosynthesis in these photoautotrophic green algae directly produced gaseous hydrogen under aerobic conditions. The amount of hydrogen produced was different for YSL01 and YSL16. Photoautotrophic hydrogen production was significantly increased when the algae grew under high CO2 conditions but the biomass-normalized hydrogen production demonstrated a limited effect in response to CO2 elevation, indicating that the increase in biomass production due to CO2 elevation enhanced photoautotrophic hydrogen production. The elevation of headspace O2 significantly decreased hydrogen production. The H2ase in these microalgae may be tolerant to high levels of oxygen and, thus, the activity of H2ase was not completely inhibited even in the presence of atmospheric oxygen levels. Potential H2ase reactivation was also observed after the exposure to 25% headspace oxygen for 48 h.
Photosynthetic organisms were previously reported to display a slow rate of H2 production owing to the oxygen sensitivity of H2ase20,21. Thus, the sensitivity of H2ase to oxygen has been implicated as a major obstacle to improving phototrophic biological hydrogen production22. However, Chader et al.23 showed that Chlorella sp. (eukaryotic microalga) produced small amounts of hydrogen with O2 partial pressure up to 15% in the headspace under mixotrophic conditions. Here we showed that novel microalgal strains (C. vulgaris YSL01 and YSL16) can produce hydrogen through photosynthesis using CO2 as the sole source of carbon with continuous illumination under aerobic conditions. The amount of hydrogen produced was strongly influenced by the initial oxygen concentration. The identification of photoautotrophic hydrogen production by eukaryotic algae under different oxygen levels (including atmospheric conditions) is ecologically important as the massive growth of algae can change the local environmental conditions in terms of H2 bioavailability in the natural aquatic system below the water surface, resulting in different oxygen regimes. The relative expression of HYDA mRNA and the specific activity of H2ase revealed that hydrogen production was facilitated by H2ase expression under atmospheric oxygen levels. This study provides evidence of a naturally evolved oxygen-tolerant H2ase in eukaryotic microalgae, and further study may open a new avenue for continuous biophotolysis to produce hydrogen and an opportunity to develop an artificial oxygen-tolerant H2ase-based biomimetic photovoltaic cell using eukaryotic algae.
Methods
Strain isolation and growth conditions
C. vulgaris strains (YSL01 and YSL16) were isolated from a local lake, Wonju, South Korea (Supplementary Fig. 4 and Supplementary Table 2). Subcultures were made by inoculating Petri plates containing EDTANa2-lacking bold basal medium (BBM) solidified with 1.5% (w/v) bacteriological agar with 50 μl of culture solution. In addition, 50 μl aliquots of the same dilutions were plated in 96-well microtiter plates containing 200 μl of EDTANa2-lacking BBM. The purity of the culture was confirmed by repeated plating and by regular observation using a microscope. Each microalgal species, with an initial optical density of 0.3–0.5 at 680 nm, was inoculated into 500 ml aluminium crimp-sealed culture bottles containing 250 ml of EDTANa2-lacking BBM. The experiments were performed in triplicate using 500 ml culture bottles with a working volume of 250 ml. The experimental bottles were incubated under white fluorescent light with an illumination of 50 μmol m−2 s−1 at 27 °C for 5 weeks with shaking at 150 r.p.m. and without organic carbon supplementation. The headspace of the culture bottles was artificially replaced with four different gas mixtures comprising N2, CO2 and O2. The initial partial pressures of both CO2 and O2 ranged from 5 to 15%, and atmospheric conditions were also investigated. The specific growth rate (μ) was calculated as described by Jiang et al.24
Hydrogen and oxygen measurements
H2 and O2 in the headspace of the serum bottles was periodically measured using a GC-14 gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a thermal conductivity detector and a molecular sieve 5A 80/100 column, using Ar as a carrier gas.
Crude cell extract and H2ase activity
The crude cell extract preparation and the measurement of H2ase activity were performed as described by Ueno et al.25 In brief, microalgae cultivated under the different O2 concentrations for 0–8 days were harvested at regular time intervals by centrifugation (12,000 g, 4 °C, 15 min) and washed twice in 20 mM phosphate buffered (pH 7.5) 1% NaCl solution. The resulting pellet was resuspended in an equal volume of the same buffer, and the cell suspension was flushed with oxygen-free nitrogen gas under anaerobic conditions. After 5 min (or 24 h, which is only for the experimental results in Supplementary Table 1) of anaerobic adaptation, the cells were harvested by centrifugation (12,000 g, 4 °C, 20 min) and resuspended in a basal buffer containing 50 mM Tris (hydroxymethyl) aminomethane–HCl (pH 8.0), 2 mM MgCl2 and 1 mM dithiothreitol, followed by the addition of powdered sodium dithionite (50 mM). The suspension was placed in a sealed steel beaker, sonicated at 300 W for 10 min in an ice-water bath under a pulsed 75%-duty-cycle condition (Sonomasher, Ulsso-Tech, Seoul, Korea) and centrifuged at 15,000 g for 20 min at 4 °C. The crude extract was prepared under strict anaerobic conditions at 4 °C. H2ase activity was quantified by the amount of hydrogen evolved from methyl viologen, which was reduced by sodium dithionite. Hydrogen production was determined by gas chromatography using a thermal conductivity detector (GC6890A, Agilent Technologies, Santa Clara, CA, USA). The assays were prepared in seal-lock vials (20 ml) with equal volumes of the liquid and gas phases. The sample (0.1–0.25 ml) was injected into 10 ml of basal buffer containing 5 mM methyl viologen and 5 mM sodium dithionite and incubated in a shaker at 28 °C for 30 min. One unit of activity was defined as the amount of H2ase needed to evolve 1 μmol of hydrogen gas per minute. The protein concentration in the crude extracts was determined by the Bradford method using BSA as a standard.
Total RNA isolation and H2ase gene detection
Total RNA was isolated from the algal cells using the RNeasy kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions26. mRNA was harvested from microalgae cultivated under different O2 concentrations for 8 days. The RNA concentration of each sample was determined by measuring the absorbance at 260 nm using a spectrophotometer. The integrity of each RNA sample was evaluated using the Agilent 2100 BioAnalyzer (Agilent Technologies). Complementary DNA synthesis was performed with 1 μg of total RNA using random primers (Invitrogen, Carlsbad, CA) and Superscript II reverse transcriptase (Invitrogen). Real-time reverse transcriptase PCR analyses were performed using a 7500 Real-Time PCR System (Applied Biosystems Inc, Foster City, CA). Reactions were performed in a 25-μl volume containing 12.5 μl of 2X SYBR Green reaction buffer, 1 μl of cDNA (corresponding to 25 ng of reverse transcribed total RNA) and 5 pmol of each HYDA-specific primer obtained from C. fusca (GenBank accession number AJ298228). After an initial incubation for 2 min at 50 °C, the cDNA was denatured at 95 °C for 10 min, followed by 45 cycles of PCR (95 °C for 15 s and 60 °C for 60 s). The data analyses were performed using 7500 system SDS software, version 1.3.1 (Applied Biosystems Inc). For the time course experiment, the samples were collected during hydrogen production, starting from 0 time under atmospheric conditions. The relative HYDA expression levels for all samples were calculated and normalized with respect to the 18S ribosomal RNA expression levels. The following primers were used: HYDA (AJ298228.1) forward, 5′-TGTCCTCCAACACCTCAGGC-3′; HYDA reverse, 5′-CTCCTGCTCTCCTTGGGCTT-3′; 18S rRNA (DQ644506.1) forward, 5′-GGCTTAATTGTCCGGGACTC-3′; and 18S rRNA reverse, 5′-AATGAAATACGAATGCCCCC-3′.
Additional information
How to cite this article: Hwang, J.-H. et al. Photoautotrophic hydrogen production by eukaryotic microalgae under aerobic conditions. Nat. Commun. 5:3234 doi: 10.1038/ncomms4234 (2014).
Accession codes
References
Embley, T. M. & Martin, W. Eukaryotic evolution, changes and challenges. Nature 440, 623–630 (2006).
Gaffron, H. & Rubin, J. Fermentative and photochemical production of hydrogen in algae. J. Gen. Physiol. 26, 219–240 (1942).
Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 177, 272–280 (2009).
Melis, A. & Happe, T. Hydrogen production. Green algae as a source of energy. Plant Physiol. 127, 740–748 (2001).
Van de Waal, D. B. et al. Reversal in competitive dominance of a toxic versus non-toxic cyanobacterium in response to rising CO2 . ISME J. 5, 1438–1450 (2011).
Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306 (2007).
Florin, L., Tsokoglou, A. & Happe, T. A novel type of iron hydrogenase in the green alga Scenedesmus obliquus is linked to the photosynthetic electron transport chain. J. Biol. Chem. 276, 6125–6132 (2001).
Melis, A. Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae). Planta 226, 1075–1086 (2007).
Ghirardi, M. L. et al. Microalgae: a green source of renewable H2 . Trends Biotechnol. 18, 506–511 (2000).
Wünschiers, R., Senger, H. & Schulz, R. Electron pathways involved in H2-metabolism in the green alga Scenedesmus obliquus. Biochim Biophys Acta 1503, 271–278 (2001).
Bothe, H., Schmitz, O., Yates, M. G. & Newton, W. E. Nitrogen fixation and hydrogen metabolism in cyanobacteria. Microbiol. Mol. Biol. Rev. 74, 529–551 (2010).
Volbeda, A. et al. Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature 373, 580–587 (1995).
Hemschemeier, A., Melis, A. & Happe, T. Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth. Res. 102, 523–540 (2009).
Allakhverdiev, S. I. et al. Photosynthetic hydrogen production. J. Photochem. Photobiol. C 11, 101–113 (2010).
Stripp, S. T. & Happe, T. How algae produce hydrogen-news from the photosynthetic hydrogenase. Dalton Trans. 45, 9960–9969 (2009).
Srirangan, K., Pyne, M. E. & Chou, C. P. Biochemical and genetic engineering strategies to enhance hydrogen production in photosynthetic algae and cyanobacteria. Bioresour. Technol. 102, 8589–8604 (2011).
Ogbonna, J. C. & Tanaka, H. Cyclic autotrophic/heterotrophic cultivation of photosynthetic cells: a method of achieving continuous cell growth under light/dark cycles. Bioresour. Technol. 65, 65–72 (1998).
Rocha, J. M. S., Garcia, J. E. C. & Henriques, M. H. F. Growth aspects of the marine microalga Nannochloropsis gaditana. Biomol. Eng. 20, 237–242 (2003).
Stripp, S. T. et al. How oxygen attacks [FeFe] hydrogenases from photosynthetic organisms. Proc. Natl Acad. Sci. USA 106, 17331–17336 (2009).
Bala, A. K. & Murugesan, A. G. Biological hydrogen production by the algal biomass Chlorella vulgaris MSU 01 strain isolated from pond sediment. Bioresour. Technol. 102, 194–199 (2011).
Yoon, J. H., Sim, S. J., Kim, M. S. & Park, T. H. High cell density culture of Anabaena variabilis using repeated injections of carbon dioxide for the production of hydrogen. Int. J. Hydrogen Energ. 27, 1265–1270 (2002).
Lubitz, W., Reijerse, E. J. & Messinger, J. Solar water-splitting into H2 and O2 design principles of photosystem II and dehydrogenases. Energy Environ. Sci. 1, 15–31 (2008).
Chader, S., Hacene, H. & Agathos, S. N. Study of hydrogen production by three strains of Chlorella isolated from the soil in the Algerian Sahara. Int. J. Hydrogen Energ. 34, 4941–4946 (2009).
Jiang, L., Luo, S., Fan, X., Yang, Z. & Guo, R. Biomass and lipid production of marine microalgae using municipal wastewater and high concentration of CO2 . Appl. Energ. 88, 3336–3341 (2011).
Ueno, Y., Kurano, N. & Miyachi, S. Purification and characterization of hydrogenase from the marine green alga, Chlorococcum littorale. FEBS Lett. 443, 144–148 (1999).
Kruse, O., Rupprecht, J., Mussgnug, J. H., Dismukes, G. C. & Hankamer, B. Photosynthesis: a blueprint for solar energy capture and biohydrogen production technologies. Photochem. Photobiol. Sci. 4, 957–970 (2005).
Acknowledgements
This work was supported by the Senior Researchers program (National Research Foundation of Korea, 2013069183), the Eco-Innovation research project (Global-Top project, 2012001090001) of the Korea Ministry of Environment, and the Korea Institute of Science & Technology (No. 2Z03860).
Author information
Authors and Affiliations
Contributions
B.-H.J. conceived the project; J.-H.H., J.-A.C. and B.-H.J. designed the experiments; J.-H.H., J.-A.C., D.P., W.L., Y.K., J.C., M.-K.J., W.J. and B.-H.J. performed the experiments; J-.H.H., H.-C.K., J.-A.C., R.A.I.A.-S., I.-H.N., S.-N.K. and B.-H.J. analysed the data; J.-H.H., H.-C.K., J.A.C., R.A.I.A.-S., B.A.D., J.M.R., J.R.K. and B.-H.J. interpreted the data; J.-H.H., H.-C.K., R.A.I.A.-S., B.A.D., J.M.R., J.R.K., H.S. and B.-H.J. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1-4 and Supplementary Tables 1-2 (PDF 1252 kb)
Rights and permissions
About this article
Cite this article
Hwang, JH., Kim, HC., Choi, JA. et al. Photoautotrophic hydrogen production by eukaryotic microalgae under aerobic conditions. Nat Commun 5, 3234 (2014). https://doi.org/10.1038/ncomms4234
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms4234
This article is cited by
-
Molecular-level architecture of Chlamydomonas reinhardtii’s glycoprotein-rich cell wall
Nature Communications (2024)
-
A mini review on microwave and contemporary based biohydrogen production technologies: a comparison
Environmental Science and Pollution Research (2022)
-
Lignocellulose, algal biomass, biofuels and biohydrogen: a review
Environmental Chemistry Letters (2021)
-
Improved photobio-H2 production regulated by artificial miRNA targeting psbA in green microalga Chlamydomonas reinhardtii
Biotechnology for Biofuels (2018)
-
Water-splitting-based, sustainable and efficient H2 production in green algae as achieved by substrate limitation of the Calvin–Benson–Bassham cycle
Biotechnology for Biofuels (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.