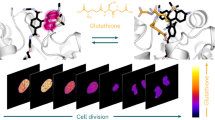
Abstract
Aqueous sulphides, including hydrogen sulphide, have important roles in biological signalling and metabolic processes. Here we develop a selective sulphide-trapping strategy involving sulphide addition to an aldehyde; the resulting hemithioacetal undergoes a Michael addition with an adjacent unsaturated acrylate ester to form a thioacetal at neutral pH in aqueous solution. Employing this new strategy, two sulphide-selective fluorescent probes, SFP-1 and SFP-2, were synthesized on the basis of two different fluorophore templates. These probes exhibit an excellent fluorescence increase and an emission maximum shift (SFP-1) in response to Na2S and H2S in a high thiol background as found under physiological conditions. We show the utility of the probes for the selective detection of sulphides, and the capacity of our probes to monitor enzymatic H2S biogenesis and image free sulphide in living cells.
Similar content being viewed by others
Introduction
Hydrogen sulphide (H2S), along with nitric oxide (NO) and carbon monoxide (CO), belongs to the gasotransmitter family of signalling molecules in biology1,2. H2S, or aqueous sulphide, elicits diverse physiological responses, including modulation of blood pressure and reduction of ischemia reperfusion injury3,4,5, exertion of anti-inflammatory effects6 and reduction of metabolic rate7. The production of H2S is catalysed by the two pyridoxal 5′-phosphate-dependent enzymes, cystathionine β-synthase (CBS)8, and cystathionine γ-lyase9, and indirectly, via a pyridoxal 5′-phosphate-independent enzyme, 3-mercaptopyruvate sulphurtransferase10.
The lack of methods for accurate measurement of H2S has limited advances in the field. As a result of the technical limitations of available methods11,12, H2S concentrations spanning 105 orders of magnitude have been reported in the literature13. Moreover, many of the commonly used methods are not amenable for application in vivo or for analysis of tissue or blood samples. Until recently, no probe was available for the detection of aqueous sulphides in vivo (while this manuscript was under review, a related probe was reported14). A major challenge is to develop molecular probes that are capable of detecting aqueous sulphides (H2S and HS− at neural pH) in the presence of other cellular molecules, in particular, millimolar concentrations of thiols found inside most cells. Recently, a thiol-maleimide reaction was employed to tune photoinduced electron transfer of a conjugated fluorophore for thiol detection and imaging15. Although effective for detection of abundant thiol molecules, this method is unable to differentiate sulphide from thiols.
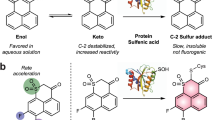
Partially inspired by this previous work, we envisioned an aromatic framework substituted by α, β-unsaturated acrylate methyl ester and aldehyde (–CHO) ortho to each other. The aldehyde group can react readily and reversibly with free sulphide to form a hemithioacetal intermediate with an exposed thiol, which is set up for a Michael addition to the proximal acrylate to yield a trapped thioacetal. This tandem reaction could tune photoinduced electron transfer of the aromatic system, thus potentially affecting fluorescence of a conjugated fluorophore. As reversible addition of a thiol to the same aldehyde yields a thioacetal that cannot perform the subsequent Michael addition step, the intermediate simply decomposes to yield the original probe, thus it will not significantly interfere sulphide detection. In addition, H2S in aqueous solution has a pKa of ~7.0, whereas thiols have higher pKa values around 8.5. Hence, aqueous sulphide is expected to be a better nucleophile at neutral pH than free thiols. In this study, we report the development of two highly sulphide-selective fluorescent probes (SFP-1 and SFP-2), using a chemical strategy for effective in vivo and in vitro detection of H2S. We demonstrate the utility of the probe in enzymatic H2S quantification and cell-based imaging applications.
Results
Synthesis and fluorescent measurements of SFP-1
We selected 1,3,5-triaryl-2-pyrazoline as the first fluorophore template because its fluorescence is sensitive to the electronic changes of the substituted aromatic groups16. During synthesis of the probe, we inadvertently obtained 1,3,5-triaryl-pyrazole because of a required oxidation step (Fig. 1). The final probe 12 (SFP-1) was characterized by nuclear magnetic resonance (NMR), mass spectrometry and X-ray crystallography (CCDC 843032; Supplementary Figs S1–S3, Supplementary Tables S1–S5, Supplementary Data 1). The phenyl substitution at C5 of the pyrazole core is designed to react with sulphide to afford 12a (Fig. 2), thus yielding a fluorescence change.
(a) MeOH, H2SO4 (2.0 equivalent), 80 °C, 12 h, 87%; (b) CrO3 (3 equiv.), AcOH (30 equiv.), Ac2O (18 equiv.) H2SO4 (4.5 equiv.) 0 °C, 1 h, 57%; (c) H2SO4, MeOH/H2O (1:1), 100 °C, 30 min, then THF, HCl, 80 °C, 2 h, 78%; (d) 1,3-propanediol (6 equiv.), p-TsOH (0.35 equiv.), anhydrous Na2SO4, 80 °C, 24 h, 80%; (e) NaBH4 (10 equiv.), 1, 4-dioxane/H2O (3:2), 65 °C, 12 h, 70%; (f) PCC (1.5 equiv.), celite, CH2Cl2, 25 °C, 1 h, 90%; (g) 3′,5′-difluoroacetophenone (1.1 equiv.), 5 N NaOH (20 equiv.), EtOH, 25 °C, 2 h, 91%; (h) HCl, THF, 25 °C, 5 h, 99%; (i) RuCl2(PPh3)3 (0.7% equiv.), HCOOH (2.8 equiv.), Et3N (1.7 equiv.), THF, 25 °C, 2 h, 83%; (j) phenylhydrazine (1.3 equiv.), HCl (1.3 equiv.), K2CO3 (0,25 equiv.), EtOH, 90 °C, 12 h, 65%; (k) PCC (5 equiv.), celite, CH2Cl2, 25 °C, 2 h, 86%; (l) Pd(OAc)2 (0.1 equiv.), methyl acrylate (1.2 equiv.), Bu4NAc (3 equiv.), K2CO3 (1.5 equiv.), KCl (1.5 equiv.), DMF, 90 °C, 2 h, 56%. DMF, N,N-dimethylformamide; Et3N, triethylamine; NaBH4, sodium borohydrid; PCC, pyridinium chlorochromate; THF, tetrahydrofuran; p-TsOH, p-toluenesulfonic acid.
(a–c) Fluorescence spectra of the SFP-1 probe (10 μM) in PBS buffer (10 mM, pH 7.4, 10% CH3CN) at 37 °C for 60 min. Excitation: 300 nm, emission: 310–550 nm. The data represent the average of three independent experiments. (a) Incubated with 50 μM Na2S after 5, 15, 30, 45, 60, 90 and 120 min. (b) Incubated with different concentrations of Na2S (10, 20, 30, 40, 50, 60 and 80 μM). (c) Incubated with various thiols at 1 mM (CYS, cysteine; BME, 2-mercaptoethanol; DTT, dithiothreitol). (d) Fluorescent detection of H2S generation by CBS. Human CBS was mixed with either homocysteine (H) or cysteine (C)+homocysteine (10 mM each). The H2S–producing activity of CBS in the presence of cysteine and homocysteine and 5 μM probe was set at 1 and the data represent the mean±s.d. of at least three independent experiments.
Reaction of probe 12 (10 μM) with Na2S (50 μM) as an aqueous sulphide source at 37 °C in PBS buffer (pH 7.4) yielded a time-dependent fluorescence increase, which was completed within 60 min (Fig. 2a,Supplementary Fig. S4). A >10-fold increase in the fluorescence intensity accompanied by a blue shift in the emission maximum from 428 to 391 nm was observed (ɛ=2,320 M−1 cm−1, Φ=0.058). Addition of sulphide most likely eliminates the quenching effects of the conjugated, unsaturated acrylate ester and aldehyde on the 5-substituted phenyl group. Consistently, a blue shift of emission indicates a break of conjugation of SFP-1 on sulphide addition. We isolated product 12a and confirmed its molecular formula by high-resolution mass spectrometry (Supplementary Fig. S3).
Next, varying concentrations of Na2S (10–50 μM) were added to the test reaction solution. The fluorescence intensity increased linearly with the concentration of Na2S up to 50 μM (Supplementary Fig. S5), and, thereafter, reached a steady state (Fig. 2b). To characterize the direct response of the probe towards H2S, the probe was added to a buffered solution that had been bubbled with H2S gas. The presence of low concentrations of H2S led to a significant fluorescence change, confirming the utility of SFP-1 for monitoring aqueous H2S (Supplementary Fig. S6). The specificity of the probe was examined by measuring its response after exposure to various thiols in PBS buffer. Strikingly, even at high concentrations (1 mM), the response of SFP-1 to any of the tested thiols was very low, exhibiting at least 50- to 100-fold selectivity towards sulphide (Fig. 2c). In addition, the emission maximum of 12a is different from those of potential thiol addition products; monitoring emission at lower wavelength should allow further distinction between sulphide versus thiol adducts. For instance, if emission can be monitored at 350 nm, the most abundant thiol in mammalian cells, glutathione, does not interfere with sulphide, thus providing potential superb selectivity for sulphide detection and imaging.
To investigate whether the probe can be used to monitor enzymatic H2S generation, we tested its efficacy with recombinant human CBS (Fig. 2d). H2S production by CBS was readily detected in the presence of millimolar concentrations of the thiol substrates, homocysteine and/or cysteine. Doubling CBS concentration or the probe concentration (from 5 to 10 μM) resulted in increased signal intensity. These results demonstrate the excellent selectivity of the probe to H2S in a high thiol background and its utility as a molecular probe for detecting H2S biogenesis in in vitro assays. This fluorescence-based assay could be readily implemented into a high throughput format for screening compound libraries for inhibitors or activators of H2S production.
Synthesis and fluorescent measurements of SFP-2
To further test the general applicability of the sulphide-trapping chemical strategy and to develop a fluorescent probe with visible-wavelength excitation and emission, we employed 4,4-Difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene (BODIPY) as the second fluorophore template owing to its high brightness and photostability17. We synthesized the BODIPY-based probe SFP-2 (20) as shown in Figure 3. The final probe 20 (SFP-2) was characterized by NMR and mass spectrometry (Supplementary Fig. S7). We expected a turn-on response of the probe after reacting with sulphide to afford 20a (Fig. 4). We also isolated product 20a and confirmed its molecular formula by high-resolution mass spectrometry (Supplementary Fig. S8). We evaluated this new SFP-2 probe (5 μM) with Na2S (50 μM) as an aqueous sulphide source at 37 °C in 20 mM PBS buffer (pH 7.0) (Fig. 4a, Supplementary Fig. S9). SFP-2 showed a >13-fold increase of the fluorescence intensity in the emission maximum at 510 nm when excited at 465 nm (ɛ=47,100 M−1 cm−1, Φ=0.208). The high optical brightness of the probe allows for detection of free sulphide without undergoing the full intensity change.
(a) RuCl2(PPh3)3 (0.7% equivalent), HCOOH (2.8 equiv.), Et3N (1.7 equiv.), THF, 25 °C, 2 h, 92%; (b) TBSCl (1.2 equiv.), imidazole (2 equiv.), DMF, 25 °C, 12 h, 99%; (c) NaBH4 (10 equiv.), 1, 4-dioxane/H2O (3:2), 65 °C, 12 h, 72%; (d) PCC (1.5 equiv.), celite, CH2Cl2, 25 °C, 1 h, 64%; (e) (1) 2,4-dimethylpyrrole (2 equiv.), TFA (one drop), CH2Cl2, 25 °C, 12 h. (2) DDQ (1 equiv.), CH2Cl2, 25 °C, 1 h. (3) DIPEA (10 equiv.), BF3·Et2O (30 equiv.), CH2Cl2, 25 °C, 2 h, 58%; (f) TBAF (1 equiv.), THF, 25 °C, 30 min, 83%; (g) PCC (5 equiv.), MgSO4, CH2Cl2, 25 °C, 30 min, 65%; (h) Pd(OAc)2 (0.1 equiv.), methyl acrylate (4 equiv.), PPh3 (0.3 equiv.), Et3N (1.5 equiv.), CH3CN, 90 °C, 12 h, 38%. DDQ, 2,3-Dichloro-5,6-dicyano-p-benzoquinone; DIPEA , N, N-Diisopropylethylamine; DMF, N,N-dimethylformamide; Et3N, triethylamine; NaBH4, sodium borohydrid; PCC, pyridinium chlorochromate; TBAF, Tetrabutylammonium fluoride trihydrate; TBSCl, tert-Butyl(chloro)dimethylsilane; TFA, Trifluoroacetic acid; THF, tetrahydrofuran.
(a–c) Fluorescence spectra of the SFP-2 probe (5 μM) in PBS buffer (20 mM, pH 7.0, 1% DMSO) at 37 °C. Excitation: 465 nm, emission: 480–580 nm. The data represent the average of three independent experiments. (a) Incubated with 50 μM Na2S after 5, 15, 30, 45, 60, 75, 90, 120, 150, 180 and 240 min. (b) Incubated with different concentrations of Na2S (5, 10, 20, 40, 60, 80 and 100 μM) for 120 min. (c) Incubated with various thiols at 1 mM (BME, 2-mercaptoethanol; CYS, cysteine; GSH, glutathione) for 180 min. (d) Incubated with 1 μl H2S buffer (bubbling H2S 10 min–saturated solution) at 25 °C from 5 s–20 min.
We further examined the sensitivity of SFP-2 for sulphide. The fluorescent intensity increased by 2.6–16 folds with addition of 5–100 μM Na2S (Fig. 4b; Supplementary Figs S10, S11). The turn-on fluorescence response is also highly selective for sulphide versus various biological relevant thiols in the PBS buffer (Fig. 4c). The SFP-2 probe is ~260-fold more selective towards Na2S than to cysteine, and ~150-fold more selective for Na2S than for glutathione. Direct response towards H2S was also tested. After addition of 1 μl H2S buffered solution (10 min H2S bubbling), a significant fluorescence increase was observed after 5 s to 20 min of mixing, and the reaction was complete in 20 min at 25 °C (Fig. 4d). A smaller amount of H2S can still induce a significant response, further confirming that SFP-2 probe is a sensitive and selective probe for H2S detection (Supplementary Fig. S12).
Cellular imaging experiments
Additionally, we tested the utility of both probes for live-cell imaging of sulphide. Even at high concentrations of SFP-1 (50 μM), adverse effects of the probe on cell viability were minimal (Supplementary Fig. S13). HeLa cells were incubated with either probe for 15 min before replacing the culture medium with fresh medium containing varying concentrations of Na2S. Whereas some background fluorescence was observed even in the absence of added sulphide for 10 μM of SFP-1 (Fig. 5a), the signal intensity increased as the concentration of sulphide was increased from 10 to 100 μM (Fig. 5b–d). SFP-2 (2 μM) responded at slightly higher concentrations of Na2S, with the sulphide concentration ranging from 0 to 200 μM (Fig. 5e–h). However, SFP-2 is a brighter probe and excites and emits at visible range that is desirable for cell-based imaging. These results demonstrate that these probes are selective for sulphide and amenable for live-cell imaging.
(a–d) Imaging of aqueous sulphide in HeLa cells after 15 min incubation using SFP-1 (12) or SFP-2 (20). For 12, excitation and data collection were performed using the corresponding filters for DAPI (blue) on a DSU spinning disk confocal. For 20, similar experiments were performed using the filter for green fluorescent protein (green). Images were obtained by using widefield fluorescence capture with SFP-1 (10 μM), with increasing concentrations of Na2S: (a) 0 μM, (b) 10 μM, (c) 50 μM, and (d) 100 μM. With SFP-2 (2 μM), confocal fluorescence capture was utilized, and Na2S concentrations were varied from (e) 0 μM, (f) 50 μM, (g) 100 μM, and (h) 200 μM. Scale bar represents 20 μm.
In addition to supplementing cells with extraneous sources of sulphide, we sought to determine whether we could detect intrinsically produced H2S by perturbing the pool of precursors to H2S biosynthesis inside the cell. The amino acid cysteine and glutathione (reduced) (GSH) can both serve as potential sulphide sources. The previously mentioned enzymes, CBS and cystathionine γ-lyase, both use cysteine as a substrate for H2S production8,9,18,19,20. GSH can be broken down by γ-glutayml transpeptidase and a dipeptidase to give cysteine21,22,23, which can then be converted to H2S. Therefore, we tested whether a perturbation of the intracellular levels of either cysteine, or GSH could result in an increased cellular concentration of H2S. Imaging experiments were carried out with SFP-2, as previously described, and cells were incubated with either 100 μM GSH or cysteine. After 30 min of incubation, addition of both thiol species elicited a significant response rivalling that observed for Na2S (Fig. 6). Other biologically relevant sulphur sources, including a thioether and a disulphide, did not generate a similar response (Supplementary Fig. S14). These results further indicate that these probes are capable of detecting not only external sulphides supplemented to cell cultures, but also sulphides biologically produced by the cells.
(a–c) Imaging of sulphide substrates in HeLa cells after 30 min incubation using SFP-2 (20). Images were obtained by using confocal fluorescence capture with 2 μM probe, with either: (a) 0 μM sulphide source, (b) 100 μM GSH, (c) 100 μM cysteine. Scale bar represents 40 μm.
To generate a significant response for both SFP-1 and SFP-2, higher concentrations of Na2S are required for the live-cell imaging experiments than the in vitro experiments. We reason that sodium sulphide, with its high charge density, could have difficulty passing through the cell membrane. The addition of this extraneous sulphide may lead to a much smaller fluctuation of the free sulphide level inside cells. With GSH and cysteine, 1 mM of either thiol gave a very weak response for SFP-1 and SFP-2 in vitro (Figs 2c and 4c), whereas only 100 μM of each elicited a significant response approximating that of Na2S in vivo. Considering the inability of the probe to detect cysteine or GSH in vitro and the millimolar concentrations of thiols already existing inside cells, we believe the response is a result of the free sulphide generated intracellularly owing to a response to the perturbed cellular levels of cysteine or GSH. We suspect that supplementing extra amounts of glutathione may disrupt glutathione homeostasis and H2S biogenesis, leading to an increased level of H2S. Thus, the probe shows great promise as a reporter for monitoring sulphide fluctuation inside cells, and could help elucidating pathways for sulphide production and uncovering new genes responsible for sulphide homeostasis.
Discussion
The development of innovative fluorescent imaging probes has revolutionized cell biology, allowing localization and dynamic monitoring of cellular metabolite and inorganic ion pools15,16,24,25,26,27,28,29. A significant bottleneck in the emerging field of H2S/aqueous sulphide signalling is the absence of technology for effective in vivo detection and imaging, a problem that is exacerbated by the high intracellular thiol concentration. In this study, we have successfully developed a chemical strategy for selective sulphide detection, which can be used to monitor sulphide generation from enzymes and for cell-based sulphide imaging in live cells in the presence of large excess of thiols. Tandem chemical reactions, consisted of a sulphide addition to an aldehyde and the resulting hemithioacetal performing a Michael addition to an unsaturated acrylate ester to form a thioacetal at neutral pH in aqueous solution, provide the basis for the sulphide selectivity. We show that the same chemistry can be readily adapted to different fluorescent templates for sulphide detection and imaging. The same chemistry will lead to new probes with faster response, which may help to monitor fluctuations of H2S in situ. Further optimization and utilization of this strategy and this class of probes should dramatically accelerate future studies of H2S in biology.
Methods
Probe synthesis
Detailed description of the synthesis of each probe can be found in the Supplementary Methods. Each step was characterized by thin-layer chromatography, high-resolution mass spectra, and both 1H and 13C NMR (Supplementary Figs S15–S54).
Fluorometric analysis
All fluorescence measurements were carried out at room temperature on a Varian Cary Eclipse fluorescence spectrophotometer. Samples were excited at 300 and 465 nm with the excitation and emission slit widths set at 5 and 10 nm for SFP-1 and SFP-2, respectively. The emission spectrum was scanned from 310 to 550 nm and from 480 to 580 nm at 120 nm min−1, respectively. The photomultiplier voltage was set at 1,000 V for SFP-1 and 600 V for SFP-2. The probe was dissolved in CH3CN or dimethylsulphoxide (DMSO) to make a 10 mM stock solution, which was diluted to the required concentration for measurement.
Cytotoxicity assay
HeLa cells were grown up in DMEM media with 10% FBS and penicillin/streptomycin (Invitrogen). Cells were allowed to grow to 80% confluency before being collected using trypsin-EDTA. The cell number was determined and solution was diluted to a final concentration of 2.22×105 cells ml−1 in the aforementioned media. A final number of 2×104 cells (90 μl) was transferred to each well in a 96-well plate (BD Falcon). Cells were incubated overnight at 37 °C in a 5% CO2 atmosphere. A serial dilution on SFP-1 was performed in DMEM media, with 10 μl added to each well to give final concentrations of 0.4, 0.8, 1.6, 3.1, 12.5, 25, 50 and 100 μM probe. Cells were allowed to incubate for 20 h. Wells containing only cells and only probe were also set up to serve as positive and negative controls.
Dye solution and stop/solubilization mix were obtained from a CellTiter 96 non-radioactive cell proliferation assay (Promega). Cytotoxicity assay was performed as per manufacturer's instructions. Absorbance at 570 was monitored using a Synergy plate reader (Biotek). Data was collected for three separate serial dilutions and averaged.
Cellular imaging experiments
HeLa cells were grown, as previously described. Cells were allowed to grow to 80% confluency before being collected and transferred to a 6-well plate (BD Falcon). These cells were allowed to grow overnight at 37 °C in a 5% CO2 atmosphere. Cells were maintained at these conditions until immediately before imaging experiments. At this time, a final concentration of 10 μM SFP-1 or 2 μM SFP-2 was added to the cells and they were allowed to incubate at the previous conditions for 15 min. Media was then removed, and fresh media was added to remove any probe left in solution and optimize the background signal. The sulphur source was then added (Na2S, cysteine, or GSH) to the desired concentration and cells were incubated for 15–30 min at room temperature before imaging.
All imaging experiments were performed on a fixed cell DSU spinning confocal microscope (Olympus). Widefield fluorescence capture was used to visualize SFP-1 under all conditions. Excitation and emission monitored using the 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) filters provided with the scope, set at 387 per 11 nm and 440 per 40 nm, respectively. Confocal fluorescence capture was used to visualize SFP-2. Excitation and emission were monitored using green filter provided with the scope, set at 485 per 20 nm and 525 per 30 nm, respectively. Imaging performed using either the X20 or X40 dry objectives that are provided with the scope. Images were captured using Slidebook software.
Additional information
How to cite this article: Qian, Y. et al. Selective fluorescent probes for live-cell monitoring of sulphide. Nat. Commun. 2:495 doi: 10.1038/ncomms1506 (2011).
References
Szabó, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 6, 917–935 (2007).
Kabil, O. & Banerjee, R. The redox biochemistry of H2S. J. Biol. Chem. 285, 21903–21907 (2010).
Yang, G. et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322, 587–590 (2008).
Elrod, J. W. et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl Acad. Sci. USA 104, 15560–15565 (2007).
Blackstone, E. & Roth, M. B. Suspended animation-like state protects mice from lethal hypoxia. Shock 27, 370–372 (2007).
Zanardo, R. C. et al. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 20, 2118–2120 (2006).
Blackstone, E., Morrison, M. & Roth, M. B. Hydrogen sulfide induces a suspended animation-like state in mice. Science 308, 518 (2005).
Singh, S., Padovani, D., Leslie, R. A., Chiku, T. & Banerjee, R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 284, 22457–22466 (2009).
Chiku, T. et al. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 284, 11601–11612 (2009).
Shibuya, N. et al. 3-mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 11, 703–714 (2009).
Jacobs, M. B., Braverman, M. M. & Hochheiser, S. Ultra-micro determination of sulphides in air. Anal. Chem. 29, 1349–1351 (1957).
Whitfield, N. L., Kreimier, E. L., Verdial, F. C., Skovgaard, N. & Olson, K. R. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, 1930–1937 (2008).
Olson, K. R. Is hydrogen sulphide a circulating 'gasotransmitter' in vertebrate blood? Biochim. Biophys. Acta 1787, 856–863 (2009).
Lippert, A. R., New, E. J. & Chang, C. J. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J. Am. Chem. Soc. 133, 10078–10080 (2011).
Yi, L. et al. A highly sensitive fluorescence probe for fast thiol-quantification assay of glutathione reductase. Angew. Chem. Int. Ed. Engl. 48, 4034–4037 (2009).
Cody, J., Mandal, S., Yang, L. & Fahrni, C. J. Differential tuning of the electron transfer parameters in 1,3,5-triarylpyrazolines: a rational design approach for optimizing the contrast ratio of fluorescent probes. J. Am. Chem. Soc. 130, 13023–13032 (2008).
Loudet, A. & Burgess, K. BODIPY dyes and their derivative: syntheses and spectroscopic properties. Chem. Rev. 107, 4891–4932 (2007).
Abe, K. & Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 16, 1066–1071 (1996).
Chen, X., Jhee, K. & Kruger, W. D. Production of the neuromodulator H2S by cystathionine β–synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 279, 52082–52086 (2004).
Wang, R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Signal. 5, 493–501 (2003).
Griffith, O. W., Bridges, R. & Meister, A. Evidence that the γ-glutamyl cycle functions in vivo using intracellular glutathione: effects of amino acids and sélective inhibition of enzymes. Proc. Natl Acad. Sci. USA 75, 5405–5408 (1978).
Kumar, T. R. et al. Reproductive defects in γ-glutamyl transpeptidase deficient mice. Endocrinology 141, 4270–4277 (2000).
Lieberman, M. W. et al. Growth retardation and cysteine deficiency in γ-glutamyl transpeptidase deficient mice. Proc. Natl Acad. Sci. USA 93, 7923–7926 (1996).
Giepmans, B. N., Adams, S. R., Ellisman, M. H. & Tsien, R. Y. The fluorescent toolbox for assessing protein locations and function. Science 312, 217–224 (2006).
McQuade, L. E. & Lippard, S. J. Fluorescent probes to investigate nitric oxide and other reactive nitrogen species in biology. Curr. Opin. Chem. Biol. 14, 43–49 (2010).
Tomat, E. & Lippard, S. J. Imaging mobile zinc in biology. Curr. Opin. Chem. Biol. 14, 225–230 (2010).
Miller, E. W. & Chang, C. J. Fluorescent probes for nitric oxide and hydrogen peroxide in cell signaling. Curr. Opin. Chem. Biol. 11, 620–625 (2007).
Que, E. L., Domaille, D. W. & Chang, C. J. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 108, 1517–1549 (2008).
Taki, M., Wolford, J. L. & O'Halloran, T. V. Emission ratiometric imaging of intracellular zinc: design of a benzoxazole fluorescent sensor and its application in two-photon microscopy. J. Am. Chem. Soc. 126, 712–713 (2004).
Acknowledgements
We thank Dr G. Jia for help with mammalian cell cultures, Dr C. Labno for assistance in all imaging experiments, Dr J. Wang for advice on the probe synthesis, and Ms S.F. Reichard for editing. This work was supported in part by the US National Science Foundation International Collaboration in Chemistry between US Investigators and their Counterparts Abroad (CHE-0922998/NSFC 20921120404 to C.H.) and the National Institutes of Health (HL58984 to R.B.). Y.Q. is partially supported by the China Scholar Program. J.K. was supported by a Chemistry-Biology Interface Predoctoral Training Grant. S.Y.Z. is supported by Natural Science Foundation of China (NSFC 20921120404).
Author information
Authors and Affiliations
Contributions
C.H. conceived the idea and directed the work. Y.Q., J.K., J.Z., O.K., R.B. and C.H. designed experiments. Y.Q. performed the synthesis and in vitro tests with help from S.Y.Z. J.K. performed cell-based imaging. O.K. performed enzymatic H2S biogenesis assay. All authors contributed to data analysis and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The University of Chicago Office of Technology and Intellectual Property is in the process of filing a patent protection of the reported probe design and the method.
Supplementary information
Supplementary Information
Supplementary Figures S1-S54, Supplementary Tables S1-S5, Supplementary Methods and Supplementary References. (PDF 2486 kb)
Supplementary Data 1
Crystallographic data for compound 12. (CIF 17 kb)
Rights and permissions
About this article
Cite this article
Qian, Y., Karpus, J., Kabil, O. et al. Selective fluorescent probes for live-cell monitoring of sulphide. Nat Commun 2, 495 (2011). https://doi.org/10.1038/ncomms1506
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/ncomms1506
This article is cited by
-
Engineered lanthanide-doped upconversion nanoparticles for biosensing and bioimaging application
Microchimica Acta (2022)
-
Recent advances of small-molecule fluorescent probes for detecting biological hydrogen sulfide
Frontiers of Chemical Science and Engineering (2022)
-
Mitochondrion-targeting near-infrared fluorescent probe for detecting intracellular nanomolar level hydrogen sulfide with high recognition rate
Analytical and Bioanalytical Chemistry (2021)
-
H2S-activatable near-infrared afterglow luminescent probes for sensitive molecular imaging in vivo
Nature Communications (2020)
-
Elucidating the excited-state dynamics behavior in near-infrared Bodipy dye and aggregates toward biophotonics
Science China Chemistry (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.