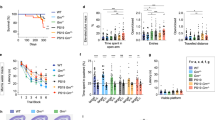
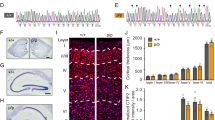
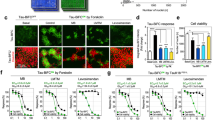
Abstract
Oligomerization of tau is a key process contributing to the progressive death of neurons in Alzheimer's disease. Tau is modified by O-linked N-acetylglucosamine (O-GlcNAc), and O-GlcNAc can influence tau phosphorylation in certain cases. We therefore speculated that increasing tau O-GlcNAc could be a strategy to hinder pathological tau-induced neurodegeneration. Here we found that treatment of hemizygous JNPL3 tau transgenic mice with an O-GlcNAcase inhibitor increased tau O-GlcNAc, hindered formation of tau aggregates and decreased neuronal cell loss. Notably, increases in tau O-GlcNAc did not alter tau phosphorylation in vivo. Using in vitro biochemical aggregation studies, we found that O-GlcNAc modification, on its own, hinders tau oligomerization. O-GlcNAc also inhibits thermally induced aggregation of an unrelated protein, TAK-1 binding protein, suggesting that a basic biochemical function of O-GlcNAc may be to prevent protein aggregation. These results also suggest O-GlcNAcase as a potential therapeutic target that could hinder progression of Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bierer, L.M. et al. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch. Neurol. 52, 81–88 (1995).
Hutton, M. et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 (1998).
Goedert, M., Jakes, R. & Crowther, R.A. Effects of frontotemporal dementia FTDP-17 mutations on heparin-induced assembly of tau filaments. FEBS Lett. 450, 306–311 (1999).
Vogelsberg-Ragaglia, V. et al. Distinct FTDP-17 missense mutations in tau produce tau aggregates and other pathological phenotypes in transfected CHO cells. Mol. Biol. Cell 11, 4093–4104 (2000).
Lewis, J. et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 25, 402–405 (2000).
Santacruz, K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005).
Alonso, A., Zaidi, T., Novak, M., Grundke-Iqbal, I. & Iqbal, K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc. Natl. Acad. Sci. USA 98, 6923–6928 (2001).
Weaver, C.L., Espinoza, M., Kress, Y. & Davies, P. Conformational change as one of the earliest alterations of tau in Alzheimer's disease. Neurobiol. Aging 21, 719–727 (2000).
Gong, C.X., Liu, F., Grundke-Iqbal, I. & Iqbal, K. Post-translational modifications of tau protein in Alzheimer's disease. J. Neural Transm. 112, 813–838 (2005).
Marcus, J.N. & Schachter, J. Targeting post-translational modifications on tau as a therapeutic strategy for Alzheimer's disease. J. Neurogenet. 25, 127–133 (2011).
Arnold, C.S. et al. The microtubule-associated protein tau is extensively modified with O-linked N-acetylglucosamine. J. Biol. Chem. 271, 28741–28744 (1996).
Lefebvre, T. et al. Evidence of a balance between phosphorylation and O-GlcNAc glycosylation of Tau proteins—a role in nuclear localization. Biochim. Biophys. Acta 1619, 167–176 (2003).
Liu, F., Iqbal, K., Grundke-Iqbal, I., Hart, G.W. & Gong, C.X. O-GlcNAcylation regulates phosphorylation of tau: a mechanism involved in Alzheimer's disease. Proc. Natl. Acad. Sci. USA 101, 10804–10809 (2004).
Kreppel, L.K., Blomberg, M.A. & Hart, G.W. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem. 272, 9308–9315 (1997).
Lubas, W.A., Frank, D.W., Krause, M. & Hanover, J.A. O-Linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J. Biol. Chem. 272, 9316–9324 (1997).
Marshall, S., Bacote, V. & Traxinger, R.R. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J. Biol. Chem. 266, 4706–4712 (1991).
Dong, D.L. & Hart, G.W. Purification and characterization of an O-GlcNAc selective N-acetyl-β-D-glucosaminidase from rat spleen cytosol. J. Biol. Chem. 269, 19321–19330 (1994).
Gao, Y., Wells, L., Comer, F.I., Parker, G.J. & Hart, G.W. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic β-N-acetylglucosaminidase from human brain. J. Biol. Chem. 276, 9838–9845 (2001).
Roquemore, E.P., Chevrier, M.R., Cotter, R.J. & Hart, G.W. Dynamic O-GlcNAcylation of the small heat shock protein αβ-crystallin. Biochemistry 35, 3578–3586 (1996).
Yuzwa, S.A. et al. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Chem. Biol. 4, 483–490 (2008).
Heiss, W.D., Szelies, B., Kessler, J. & Herholz, K. Abnormalities of energy metabolism in Alzheimer's disease studied with PET. Ann. NY Acad. Sci. 640, 65–71 (1991).
Drzezga, A. et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer's disease: a PET follow-up study. Eur. J. Nucl. Med. Mol. Imaging 30, 1104–1113 (2003).
Mielke, R., Herholz, K., Grond, M., Kessler, J. & Heiss, W.D. Clinical deterioration in probable Alzheimer's disease correlates with progressive metabolic impairment of association areas. Dementia 5, 36–41 (1994).
Shulman, J.M. et al. Functional screening of Alzheimer pathology genome-wide association signals in Drosophila. Am. J. Hum. Genet. 88, 232–238 (2011).
Liu, Y., Liu, F., Grundke-Iqbal, I., Iqbal, K. & Gong, C.X. Brain glucose transporters, O-GlcNAcylation and phosphorylation of tau in diabetes and Alzheimer's disease. J. Neurochem. 111, 242–249 (2009).
Lefebvre, T. et al. Dysregulation of the nutrient/stress sensor O-GlcNAcylation is involved in the etiology of cardiovascular disorders, type-2 diabetes and Alzheimer's disease. Biochim. Biophys. Acta 1800, 67–79 (2010).
Steen, E. et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease—is this type 3 diabetes? J. Alzheimers Dis. 7, 63–80 (2005).
Li, X., Lu, F., Wang, J.Z. & Gong, C.X. Concurrent alterations of O-GlcNAcylation and phosphorylation of tau in mouse brains during fasting. Eur. J. Neurosci. 23, 2078–2086 (2006).
Liu, F. et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer's disease. Brain 132, 1820–1832 (2009).
Schubert, D. Glucose metabolism and Alzheimer's disease. Ageing Res. Rev. 4, 240–257 (2005).
Robertson, L.A., Moya, K.L. & Breen, K.C. The potential role of tau protein O-glycosylation in Alzheimer's disease. J. Alzheimers Dis. 6, 489–495 (2004).
Wang, Z. et al. Enrichment and site mapping of O-linked N-acetylglucosamine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol. Cell. Proteomics 9, 153–160 (2010).
Yuzwa, S.A. et al. Mapping O-GlcNAc modification sites on tau and generation of a site-specific O-GlcNAc tau antibody. Amino Acids 40, 857–868 (2011).
Macauley, M.S., Shan, X., Yuzwa, S.A., Gloster, T.M. & Vocadlo, D.J. Elevation of global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis. Chem. Biol. 17, 949–958 (2010).
Uno, Y. et al. Efficacy of a novel, orally active GSK-3 inhibitor 6-methyl-N-[3-[[3-(1-methylethoxy)propyl]carbamoyl]-1H-pyrazol-4-yl]pyridine-3-carboxamide in tau transgenic mice. Brain Res. 1296, 148–163 (2009).
Le Corre, S. et al. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc. Natl. Acad. Sci. USA 103, 9673–9678 (2006).
Sahara, N. et al. Assembly of tau in transgenic animals expressing P301L tau: alteration of phosphorylation and solubility. J. Neurochem. 83, 1498–1508 (2002).
Smet-Nocca, C. et al. Identification of O-GlcNAc sites within peptides of the Tau protein and their impact on phosphorylation. Mol. Biosyst. 7, 1420–1429 (2011).
Reynolds, M.R. et al. Tau nitration occurs at tyrosine 29 in the fibrillar lesions of Alzheimer's disease and other tauopathies. J. Neurosci. 26, 10636–10645 (2006).
de Calignon, A. et al. Caspase activation precedes and leads to tangles. Nature 464, 1201–1204 (2010).
Chirita, C.N., Congdon, E.E., Yin, H. & Kuret, J. Triggers of full-length tau aggregation: a role for partially folded intermediates. Biochemistry 44, 5862–5872 (2005).
Goedert, M. et al. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature 383, 550–553 (1996).
Wilson, D.M. & Binder, L.I. Free fatty acids stimulate the polymerization of tau and amyloid beta peptides. In vitro evidence for a common effector of pathogenesis in Alzheimer's disease. Am. J. Pathol. 150, 2181–2195 (1997).
Friedhoff, P., Schneider, A., Mandelkow, E.M. & Mandelkow, E. Rapid assembly of Alzheimer-like paired helical filaments from microtubule-associated protein tau monitored by fluorescence in solution. Biochemistry 37, 10223–10230 (1998).
Taniguchi, T. et al. Effects of different anti-tau antibodies on tau fibrillogenesis: RTA-1 and RTA-2 counteract tau aggregation. FEBS Lett. 579, 1399–1404 (2005).
Clarke, A.J. et al. Structural insights into mechanism and specificity of O-GlcNAc transferase. EMBO J. 27, 2780–2788 (2008).
Conner, S.H. et al. TAK1-binding protein 1 is a pseudophosphatase. Biochem. J. 399, 427–434 (2006).
Hanson, S.R. et al. The core trisaccharide of an N-linked glycoprotein intrinsically accelerates folding and enhances stability. Proc. Natl. Acad. Sci. USA 106, 3131–3136 (2009).
Culyba, E.K. et al. Protein native-state stabilization by placing aromatic side chains in N-glycosylated reverse turns. Science 331, 571–575 (2011).
Pickhardt, M. et al. Anthraquinones inhibit tau aggregation and dissolve Alzheimer's paired helical filaments in vitro and in cells. J. Biol. Chem. 280, 3628–3635 (2005).
Acknowledgements
We thank the Scottish Rite Charitable Foundation, the Canadian Institutes of Health Research (CIHR) and the Alzheimer Society of Canada for financial support of this research. The Canada Research Chairs program, the Michael Smith Foundation for Health Research (MSFHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC, E.W.R. Steacie Award) are thanked for awards to D.J.V. NSERC is thanked for an award to S.A.Y. The MSFHR and the CIHR are thanked for awards to X.S. The MSFHR, NSERC and Human Frontier Science Program are thanked for awards to M.S.M. We thank the staff at the animal care facility at Simon Fraser University for assistance with the animal studies. P. Davies (Albert Einstein College of Medicine) is thanked for the kind gift of the CP27 and PHF-1 antibodies used in this work. Y. Deng, R. Kaul and E. McEachern are thanked for assistance with preliminary studies, large-scale synthesis of thiamet-G and productive discussion.
Author information
Authors and Affiliations
Contributions
D.J.V. directed the study. S.A.Y., X.S., M.S.M. and D.J.V. designed the experiments. X.S. conducted the rotarod assessment and the JNPL3 IHC. S.A.Y. conducted the cage hang test and the JNPL3 tau biochemistry and tau in vitro aggregation experiments. M.S.M. conducted the sTAB1 experiments. Y.S., T.C. and K.V. conducted the tau mass spectrometry. S.A.Y., X.S. and D.J.V. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.A.Y., M.S.M., X.S. and D.J.V. are eligible to receive royalties from Simon Fraser University. D.J.V. is a founder, shareholder, consultant and member of the scientific advisory board of Alectos Therapeutics.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Methods (PDF 2058 kb)
Rights and permissions
About this article
Cite this article
Yuzwa, S., Shan, X., Macauley, M. et al. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat Chem Biol 8, 393–399 (2012). https://doi.org/10.1038/nchembio.797
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.797
This article is cited by
-
Ogt-mediated O-GlcNAcylation inhibits astrocytes activation through modulating NF-κB signaling pathway
Journal of Neuroinflammation (2023)
-
Tau and neuroinflammation in Alzheimer’s disease: interplay mechanisms and clinical translation
Journal of Neuroinflammation (2023)
-
O-GlcNAcylation determines the translational regulation and phase separation of YTHDF proteins
Nature Cell Biology (2023)
-
O-GlcNAcylation regulates neurofilament-light assembly and function and is perturbed by Charcot-Marie-Tooth disease mutations
Nature Communications (2023)
-
In silico analysis of the possible crosstalk between O-linked β-GlcNAcylation and phosphorylation sites of Disabled 1 adaptor protein in vertebrates
Amino Acids (2023)