Abstract
Releasing content from large vesicles measuring several micrometres in diameter poses exceptional challenges to the secretory system. An actomyosin network commonly coats these vesicles, and is thought to provide the necessary force mediating efficient cargo release. Here we describe the spatial and temporal dynamics of the formation of this actomyosin coat around large vesicles and the resulting vesicle collapse, in live Drosophila melanogaster salivary glands. We identify the Formin family protein Diaphanous (Dia) as the main actin nucleator involved in generating this structure, and uncover Rho as an integrator of actin assembly and contractile machinery activation comprising this actomyosin network. High-resolution imaging reveals a unique cage-like organization of myosin II on the actin coat. This myosin arrangement requires branched-actin polymerization, and is critical for exerting a non-isotropic force, mediating efficient vesicle contraction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Miklavc, P. et al. Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles. J. Cell Sci. 128, 1193–1203 (2015).
Geron, E., Schejter, E. D. & Shilo, B. Z. Directing exocrine secretory vesicles to the apical membrane by actin cables generated by the formin mDia1. Proc. Natl Acad. Sci. USA 110, 10652–10657 (2013).
Nightingale, T. D. et al. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. J. Cell Biol. 194, 613–629 (2011).
Nightingale, T. D., Cutler, D. F. & Cramer, L. P. Actin coats and rings promote regulated exocytosis. Trends Cell Biol. 22, 329–337 (2012).
Masedunskas, A., Porat-Shliom, N. & Weigert, R. Linking differences in membrane tension with the requirement for a contractile actomyosin scaffold during exocytosis in salivary glands. Commun. Integr. Biol. 5, 84–87 (2012).
Porat-Shliom, N., Milberg, O., Masedunskas, A. & Weigert, R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell. Mol. Life Sci. 70, 2099–2121 (2013).
Miklavc, P. et al. Actin coating and compression of fused secretory vesicles are essential for surfactant secretion–a role for Rho, formins and myosin II. J. Cell Sci. 125, 2765–2774 (2012).
Masedunskas, A. et al. Role for the actomyosin complex in regulated exocytosis revealed by intravital microscopy. Proc. Natl Acad. Sci. USA 108, 13552–13557 (2011).
Yu, H. Y. & Bement, W. M. Multiple myosins are required to coordinate actin assembly with coat compression during compensatory endocytosis. Mol. Biol. Cell 18, 4096–4105 (2007).
Bhat, P. & Thorn, P. Myosin 2 maintains an open exocytic fusion pore in secretory epithelial cells. Mol. Biol. Cell 20, 1795–1803 (2009).
Biyasheva, A., Do, T. V., Lu, Y., Vaskova, M. & Andres, A. J. Glue secretion in the Drosophila salivary gland: a model for steroid-regulated exocytosis. Dev. Biol. 231, 234–251 (2001).
Miklavc, P., Wittekindt, O. H., Felder, E. & Dietl, P. Ca2+-dependent actin coating of lamellar bodies after exocytotic fusion: a prerequisite for content release or kiss-and-run. Ann. N. Y. Acad. Sci. 1152, 43–52 (2009).
Sokac, A. M., Co, C., Taunton, J. & Bement, W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat. Cell Biol. 5, 727–732 (2003).
Nemoto, T., Kojima, T., Oshima, A., Bito, H. & Kasai, H. Stabilization of exocytosis by dynamic F-actin coating of zymogen granules in pancreatic acini. J. Biol. Chem. 279, 37544–37550 (2004).
Rousso, T., Shewan, A. M., Mostov, K. E., Schejter, E. D. & Shilo, B. Z. Apical targeting of the formin Diaphanous in Drosophila tubular epithelia. eLife 2, e00666 (2013).
Afshar, K., Stuart, B. & Wasserman, S. A. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development 127, 1887–1897 (2000).
Morin, X., Daneman, R., Zavortink, M. & Chia, W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl Acad. Sci. USA 98, 15050–15055 (2001).
Royou, A., Field, C., Sisson, J. C., Sullivan, W. & Karess, R. Reassessing the role and dynamics of nonmuscle myosin II during furrow formation in early Drosophila embryos. Mol. Biol. Cell 15, 838–850 (2004).
Bresnick, A. R. Molecular mechanisms of nonmuscle myosin-II regulation. Curr. Opin. Cell Biol. 11, 26–33 (1999).
Dawes-Hoang, R. E. et al. Folded gastrulation, cell shape change and the control of myosin localization. Development 132, 4165–4178 (2005).
Spiering, D. & Hodgson, L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adhes. Migr. 5, 170–180 (2011).
Munjal, A., Philippe, J. M., Munro, E. & Lecuit, T. A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 524, 351–355 (2015).
Goley, E. D. & Welch, M. D. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7, 713–726 (2006).
Sanderson, J. M. Resolving the kinetics of lipid, protein and peptide diffusion in membranes. Mol. Membr. Biol. 29, 118–143 (2012).
Yu, H. Y. & Bement, W. M. Control of local actin assembly by membrane fusion-dependent compartment mixing. Nat. Cell Biol. 9, 149–159 (2007).
Dean, S. O., Rogers, S. L., Stuurman, N., Vale, R. D. & Spudich, J. A. Distinct pathways control recruitment and maintenance of myosin II at the cleavage furrow during cytokinesis. Proc. Natl Acad. Sci. USA 102, 13473–13478 (2005).
Ideses, Y., Sonn-Segev, A., Roichman, Y. & Bernheim-Groswasser, A. Myosin II does it all: assembly, remodeling, and disassembly of actin networks are governed by myosin II activity. Soft Matter 9, 7127–7137 (2013).
Levayer, R. & Lecuit, T. Biomechanical regulation of contractility: spatial control and dynamics. Trends Cell Biol. 22, 61–81 (2012).
Kasza, K. E. & Zallen, J. A. Dynamics and regulation of contractile actin-myosin networks in morphogenesis. Curr. Opin. Cell Biol. 23, 30–38 (2011).
Wilson, C. A. et al. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature 465, 373–377 (2010).
Medeiros, N. A., Burnette, D. T. & Forscher, P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 8, 215–226 (2006).
Carvalho, K. et al. Cell-sized liposomes reveal how actomyosin cortical tension drives shape change. Proc. Natl Acad. Sci. USA 110, 16456–16461 (2013).
Carvalho, K. et al. Actin polymerization or myosin contraction: two ways to build up cortical tension for symmetry breaking. Phil. Trans. R. Soc. B 368, 20130005 (2013).
Xu, N., Bagumian, G., Galiano, M. & Myat, M. M. Rho GTPase controls Drosophila salivary gland lumen size through regulation of the actin cytoskeleton and moesin. Development 138, 5415–5427 (2011).
Von Stein, W., Ramrath, A., Grimm, A., Muller-Borg, M. & Wodarz, A. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development 132, 1675–1686 (2005).
Rauzi, M., Lenne, P. F. & Lecuit, T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114 (2010).
Acknowledgements
We thank J. M. Philippe and T. Lecuit for the active Rho1 reporter strain, and the Bloomington and VDRC stock centres for Drosophila lines. We thank S. Levin-Zaidman (Irving and Cherna Moskowitz Center for Nano and Bio-Nano Imaging, Weizmann Institute of Science) for conducting the electron microscopy studies. We thank E. Bouchbinder and A. Livne for discussions and suggestions on biomechanics and quantification of vesicle secretion. We are grateful to members of the B.-Z.S. laboratory, especially E. Geron, for stimulating discussions. This work was supported by a USA-Israel BSF grant to E.D.S. and B.-Z.S. B.-Z.S. is an incumbent of the Hilda and Cecil Lewis chair in Molecular Genetics.
Author information
Authors and Affiliations
Contributions
T.R. designed the experiments, carried them out and participated in writing the manuscript, E.D.S. and B.-Z.S. designed the experiments and participated in writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
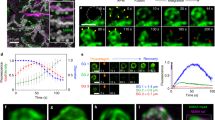
Supplementary Figure 1 Fusion and membrane mixing in glue-vesicle secretion.
(a–a ′) Time series of a single vesicle secretion event from a glue-secreting salivary gland expressing UAS-LifeAct-GFP under the fkh-Gal4 driver (green, a ′), and injected with Dextran-TMR (red, a). At t = 0, Dextran is restricted to the lumen by the apical membrane, outlined by actin. Dextran is observed to fill the vesicle prior to appearance of an actin coat, demonstrating that fusion precedes coating. (b–b ′) Time series of a single vesicle from a glue-secreting salivary gland expressing both the PI(4,5)P2 sensor UAS-PH-PLCδ-GFP (green, b), and UAS-LifeAct-Ruby (red, b ′) under the fkh-Gal4 driver. In addition to a strong apical membrane localization, PI(4,5)P2 begins to outline the vesicle at 0.32 m, ahead of actin coating, first observed at 0.97 m. (c–c ′) Time series of a single vesicle, from glue-secreting salivary glands expressing the PI(4,5)P2 sensor PH-PLCδ-GFP under the fkh-Gal4 driver (c ′), and injected with Dextran-TMR (c). Dextran is restricted to the lumen by the apical membrane, outlined by PI(4,5)P2. PI(4,5)P2 appears upon the vesicle membrane simultaneously with the appearance of Dextran (at 0.14 m). Scale bars: 5 μm.
Supplementary Figure 2 The number of myosin stripes is weakly correlated with vesicle size.
A graph describing the number of myosin stripes counted for each vesicle, relative to vesicle size (diameter). As demonstrated by the correlation coefficient (0.41, P-value = 0.007, Microsoft Excel), the two are only weakly correlated. n = 38 vesicles.
Supplementary information
Supplementary Information
Supplementary Information (PDF 396 kb)
Glue secretion from ecdysone treated larval salivary glands.
Time-lapse movie of cultured salivary glands expressing UAS-LifeAct-Ruby (red) under the fkh-Gal4 driver, and Sgs3-GFP under endogenous regulatory sequences (green). Movie begins 2 h after the addition of ecdysone to the culture media. GFP is observed within glue granules spanning the entire volume of the cell, and actin is observed enriched at the apical surface. An increase in GFP fluorescence in the lumen is readily apparent over time, concomitant with actin coat structures around apical vesicles, and expansion of the lumen. Movie imaged using 20× lens Plan-Apochromat NA 0.8 with 1.5 digital zoom. Stacks of 6 z planes at 4 μm intervals were taken, with no time interval. Movie was processed post imaging, and the time elapsed is shown within the movie. Scale bar: 20 μm. (MOV 1949 kb)
Hydrostatic pressure in glue vesicles following fusion.
Time-lapse movie of cultured salivary glands expressing UAS-LifeAct-Ruby (red) under the fkh-Gal4 driver, and Sgs3-GFP under endogenous regulatory sequences (green), approximately 1.5 h following secretion onset (3.5 h following addition of ecdysone). The vesicle in the center increases in size and GFP intensity just before it coats with actin and squeezes, indicative of glue that was compressed within the vesicle upon fusion. The lumen is at a deeper focal plane, and the direction of secretion is perpendicular to this optical plane. Movie imaged using 63× lens Plan-Apochromat NA 1.4 with 4 digital zoom, with no time interval. Movie was processed post imaging and the time elapsed is shown within the movie. Scale bar: 2 μm. (AVI 146 kb)
dia mutants exhibit abnormal secretion with no actin coat formation.
Time-lapse movies shown side by side of wildtype (left) and dia5/Df (right) salivary glands expressing the microfilament binding construct Utrophin-GFP under the fkh-Gal4driver. Movie begins 2 h after the addition of ecdysone to the culture media. Wildtype glands exhibit a dynamic appearance of actin coats as the lumen expands, in contrast to dia mutant salivary glands which show no actin coats, and only limited expansion of the lumen. Movies were taken simultaneously using 20× lens Plan-Apochromat NA 0.8 with 1 digital zoom. Stacks of 6 z planes at 4 μm intervals were taken, with no time interval. Movies were processed post imaging and the time elapsed is shown within the movie. Scale bar: 20 μm. (AVI 3654 kb)
zip-RNAi expressing salivary glands exhibit actin coated vesicles that do not contract.
Time-lapse movie of salivary glands expressing zip-RNAi and UAS-LifeAct-Ruby (red) under the fkh-Gal4 driver, and Sgs3-GFP under endogenous regulatory sequences (green). The population of 62 vesicles in this video was analyzed: actin coats are clearly shown forming around glue vesicles, however very few vesicles contract, as monitored by size reduction. The majority of vesicles (60%) lose their actin coat after a few minutes while glue remains within the vesicle and no size reduction is observed (red arrow). Some vesicles (15%) exhibit oscillations in actin assembly with no apparent size reduction or glue secretion (white arrows). Only a fraction of the vesicles exhibit size reduction, however for some of these vesicles fusion and content release to another vesicle take place, rather than to the lumen (purple arrow). Movie imaged using 20× lens Plan-Apochromat NA 0.8 with 1.1 digital zoom. Stacks of 7 z planes at 4 μm intervals were taken, with no time interval. Movie was processed post imaging and shows a single focal plane. The time is shown within the movie. Scale bar: 10 μm. (AVI 4053 kb)
Rok-RNAi expressing salivary glands exhibit actin coated vesicles that do not contract.
Time-lapse movie of salivary glands expressing Rok-RNAi and UAS-LifeAct-GFP under the fkh-Gal4 driver. The population of 58 vesicles in this video was analyzed: actin coats are clearly shown forming around glue vesicles, however very few vesicles contract, as monitored by size reduction. The majority of vesicles (64%) lose their actin coat after a few minutes while glue remains within the vesicle and no size reduction is observed (red arrow). Some vesicles (31%) exhibit oscillations in actin assembly with no apparent size reduction or glue secretion (white arrows). Only a small fraction of the vesicles exhibit size reduction, however for some of these vesicles fusion and content release to another vesicle take place, rather than to the lumen (purple arrow). Movie imaged using 20× lens Plan-Apochromat NA 0.5 with 1.2 digital zoom. Stacks of 10 z planes at 4 μm intervals were taken, with no time interval. Movie was processed post imaging and shows a single focal plane. The time is shown within the movie. Scale bar: 10 μm. (AVI 8612 kb)
Rho-RNAi expressing salivary glands exhibit no actin coats and formation of giant vesicles.
Time-lapse movie of glue-secreting salivary glands expressing Rho1-RNAi and the microfilament binding construct UAS-Utrophin-GFP under the fkh-Gal4 driver, and Sgs3-GFP under endogenous regulatory sequences. The movie begins 2 h after the addition of ecdysone to the culture media. Actin coats are not observed around glue vesicles. Weak actin coats are observed around giant vesicles and a few normal-sized vesicles, which are not secreted. The formation and growth of giant vesicles is readily observed over time. These glands exhibit a widened lumen, due to an early effect on gland cell morphology15,34, unrelated to secretion. Movie imaged using 20× lens Plan-Apochromat NA 0.8 with 1.5 digital zoom. Stacks of 6 z planes at 4 μm intervals were taken, with no time interval. Movie was processed post imaging and the time is shown within the movie. Scale bar: 20 μm. (MOV 416 kb)
Giant vesicles in Rho1-RNAi expressing glands are the result of fusion between multiple glue vesicles.
Time-lapse movie of salivary glands expressing Rho1-RNAi under the fkh-Gal4 driver, and Sgs3-GFP under endogenous regulatory sequences, at 60 m following secretion onset (3 h following addition of ecdysone). Actin coated vesicles are clearly seen fusing with the giant glue vesicle. The lumen is in a deeper focal plane, and the direction of secretion is perpendicular to this optical plane. Movie imaged using 63× lens Plan-Apochromat NA 1.4 with 1.5 digital zoom, with no time interval. Movie was processed post imaging, and the time elapsed is shown within the movie. Scale bar: 5 μm. (AVI 462 kb)
Rights and permissions
About this article
Cite this article
Rousso, T., Schejter, E. & Shilo, BZ. Orchestrated content release from Drosophila glue-protein vesicles by a contractile actomyosin network. Nat Cell Biol 18, 181–190 (2016). https://doi.org/10.1038/ncb3288
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3288
This article is cited by
-
Membrane transformations of fusion and budding
Nature Communications (2024)
-
Patterning of the cell cortex by Rho GTPases
Nature Reviews Molecular Cell Biology (2024)
-
Male-female communication enhances release of extracellular vesicles leading to high fertility in Drosophila
Communications Biology (2022)
-
The steroid-hormone ecdysone coordinates parallel pupariation neuromotor and morphogenetic subprograms via epidermis-to-neuron Dilp8-Lgr3 signal induction
Nature Communications (2021)
-
Interaction of microtubules and actin during the post-fusion phase of exocytosis
Scientific Reports (2019)