Abstract
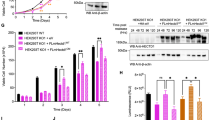
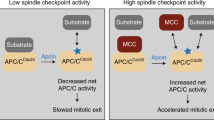
The anaphase-promoting complex (APC/C), a ubiquitin ligase, is the target of the spindle-assembly checkpoint (SAC), and it ubiquitylates protein substrates whose degradation regulates progress through mitosis1,2,3. The identity of the ubiquitin-conjugating (E2) enzymes that work with the APC/C is unclear. In an RNA interference (RNAi) screen for factors that modify release from drug-induced SAC activation, we identified the E2 enzyme UBE2S as an APC/C auxiliary factor that promotes mitotic exit. UBE2S is dispensable in a normal mitosis, but its depletion prolongs drug-induced mitotic arrest and suppresses mitotic slippage. In vitro, UBE2S elongates ubiquitin chains initiated by the E2 enzymes UBCH10 and UBCH5, enhancing the degradation of APC/C substrates by the proteasome. Indeed, following release from SAC-induced mitotic arrest, UBE2S-depleted cells neither degrade crucial APC/C substrates, nor silence this checkpoint, whereas bypassing the SAC through BUBR1 depletion or Aurora-B inhibition negates the requirement for UBE2S. Thus, UBE2S functions with the APC/C in a two-step mechanism to control substrate ubiquitylation that is essential for mitotic exit after prolonged SAC activation, providing a new model for APC/C function in human cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Musacchio, A. & Salmon, E. D. The spindle-assembly checkpoint in space and time. Nature Rev. Mol. Cell Biol. 8, 379–393 (2007).
Kim, S. H., Lin, D. P., Matsumoto, S., Kitazono, A. & Matsumoto, T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science 279, 1045–1047 (1998).
Nilsson, J., Yekezare, M., Minshull, J. & Pines, J. The APC/C maintains the spindle assembly checkpoint by targeting Cdc20 for destruction. Nature Cell Biol. 10, 1411–1420 (2008).
Pines, J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 16, 55–63 (2006).
Peters, J. M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nature Rev. Mol. Cell Biol. 7, 644–656 (2006).
Tang, Z. et al. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol. Biol. Cell 12, 3839–3851 (2001).
Aristarkhov, A. et al. E2-C, a cyclin-selective ubiquitin carrier protein required for the destruction of mitotic cyclins. Proc. Natl Acad. Sci. USA 93, 4294–4299 (1996).
Yu, H., King, R. W., Peters, J. M. & Kirschner, M. W. Identification of a novel ubiquitin-conjugating enzyme involved in mitotic cyclin degradation. Curr. Biol. 6, 455–466 (1996).
Seino, H., Kishi, T., Nishitani, H. & Yamao, F. Two ubiquitin-conjugating enzymes, UbcP1/Ubc4 and UbcP4/Ubc11, have distinct functions for ubiquitination of mitotic cyclin. Mol. Cell Biol. 23, 3497–3505 (2003).
Osaka, F., Seino, H., Seno, T. & Yamao, F. A ubiquitin-conjugating enzyme in fission yeast that is essential for the onset of anaphase in mitosis. Mol. Cell Biol. 17, 3388–3397 (1997).
Rodrigo-Brenni, M. C. & Morgan, D. O. Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell 130, 127–139 (2007).
Mathe, E. et al. The E2-C vihar is required for the correct spatiotemporal proteolysis of cyclin B and itself undergoes cyclical degradation. Curr. Biol. 14, 1723–1733 (2004).
Walker, A., Acquaviva, C., Matsusaka, T., Koop, L. & Pines, J. UbcH10 has a rate-limiting role in G1 phase but might not act in the spindle checkpoint or as part of an autonomous oscillator. J. Cell Sci. 121, 2319–2326 (2008).
Rape, M. & Kirschner, M. W. Autonomous regulation of the anaphase-promoting complex couples mitosis to S-phase entry. Nature 432, 588–595 (2004).
Thrower, J. S., Hoffman, L., Rechsteiner, M. & Pickart, C. M. Recognition of the polyubiquitin proteolytic signal. EMBO J. 19, 94–102 (2000).
Rieder, C. L. & Maiato, H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 7, 637–651 (2004).
Gascoigne, K. E. & Taylor, S. S. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell 14, 111–122 (2008).
Brito, D. A. & Rieder, C. L. Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr. Biol. 16, 1194–1200 (2006).
Kapoor, T. M., Mayer, T. U., Coughlin, M. L. & Mitchison, T. J. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 150, 975–988 (2000).
Mayer, T. U. et al. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286, 971–974 (1999).
DeBonis, S. et al. In vitro screening for inhibitors of the human mitotic kinesin Eg5 with antimitotic and antitumor activities. Mol. Cancer Ther. 3, 1079–1090 (2004).
Gartner, M. et al. Development and biological evaluation of potent and specific inhibitors of mitotic Kinesin Eg5. Chembiochem 6, 1173–1177 (2005).
den Elzen, N. & Pines, J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153, 121–136 (2001).
Hames, R. S., Wattam, S. L., Yamano, H., Bacchieri, R. & Fry, A. M. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J. 20, 7117–7127 (2001).
Brito, D. A., Yang, Z. & Rieder, C. L. Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J. Cell Biol. 182, 623–629 (2008).
Choi, E. et al. BubR1 acetylation at prometaphase is required for modulating APC/C activity and timing of mitosis. EMBO J. (2009).
Herzog, F. et al. Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science 323, 1477–1481 (2009).
Ditchfield, C. et al. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J. Cell Biol. 161, 267–280 (2003).
Bassermann, F. et al. The Cdc14B-Cdh1-Plk1 axis controls the G2 DNA-damage-response checkpoint. Cell 134, 256–267 (2008).
Stegmeier, F. et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature 446, 876–881 (2007).
Song, L. & Rape, M. Reverse the curse - the role of deubiquitination in cell cycle control. Curr. Opin. Cell Biol. 20, 156–163 (2008).
Hagting, A., Karlsson, C., Clute, P., Jackman, M. & Pines, J. MPF localization is controlled by nuclear export. EMBO J. 17, 4127–4138 (1998).
Acknowledgements
We thank P. Lehner (Cambridge Institute of Medical Research) for providing the ubiquitin-proteasome siRNA library, the Newton Trust for funding its purchase, and L. Passmore for helpful discussions. M.J.G. was supported by a Canadian Institute of Health Research fellowship, J.M., by a FEBS fellowship and C.G., by a Churchill Foundation Scholarship. M.J.G., C.G. and P.R. were also supported by a UK Medical Research Council (MRC) grant to A.R.V., and J.W. and by a Wellcome Trust grant to A.R.V. Work in J.P.'s laboratory is supported by Cancer Research UK, and in A.R.V.'s, by the MRC.
Author information
Authors and Affiliations
Contributions
M.J.G., A.R.V. and P.R. designed the siRNA screen; M.J.G. and C.G. performed the screen; M.J.G, C.G., P.R. and J.W. analysed the screen data. M.J.G. performed the analysis of UBE2S cellular function. J.M. performed the APC/C in vitro activity assays, and T.M., the cyclin-B1 degradation assays and microinjection studies. M.J.G. and T.M. performed the time-lapse studies, which J.W. helped quantify. All authors analysed and interpreted the data. The manuscript was written by M.J.G. and A.R.V.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1280 kb)
Supplementary Information
Supplementary Table 1 (XLS 70 kb)
Supplementary Information
Supplementary Table 2 (XLS 249 kb)
Rights and permissions
About this article
Cite this article
Garnett, M., Mansfeld, J., Godwin, C. et al. UBE2S elongates ubiquitin chains on APC/C substrates to promote mitotic exit. Nat Cell Biol 11, 1363–1369 (2009). https://doi.org/10.1038/ncb1983
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1983
This article is cited by
-
Weakened APC/C activity at mitotic exit drives cancer vulnerability to KIF18A inhibition
The EMBO Journal (2024)
-
Molecular and biological factors in the prognosis of head and neck squamous cell cancer
Molecular Biology Reports (2023)
-
UBE2S promotes the progression and Olaparib resistance of ovarian cancer through Wnt/β-catenin signaling pathway
Journal of Ovarian Research (2021)
-
UBE2S promotes cell chemoresistance through PTEN-AKT signaling in hepatocellular carcinoma
Cell Death Discovery (2021)
-
Single-cell transcriptome profiling reveals intratumoural heterogeneity and malignant progression in retinoblastoma
Cell Death & Disease (2021)