Abstract
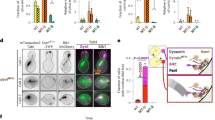
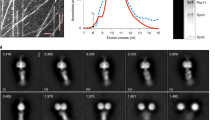
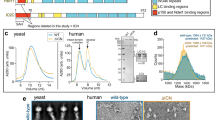
Dynein is a minus-end-directed microtubule motor with critical roles in mitosis, membrane transport and intracellular transport. Several proteins regulate dynein activity, including dynactin1, LIS1 (refs 2, 3) and NudEL (NudE-like)2,4,5,6,7,8. Here, we identify a NUDEL homologue in budding yeast and name it Ndl1. The ndl1Δ null mutant shows decreased targeting of dynein to microtubule plus ends, an essential element of the model for dynein function. We find that Ndl1 regulates dynein targeting through LIS1, with which it interacts biochemically, but not through CLIP170, another plus-end protein involved in dynein targeting9. Ndl1 is found at far fewer microtubule ends than are LIS1 and dynein. However, when Ndl1 is present at a plus end, the molar amount of Ndl1 approaches that of LIS1 and dynein. We propose a model in which Ndl1 binds transiently to the plus end to promote targeting of LIS1, which cooperatively recruits dynein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schroer, T. A. Dynactin. Annu. Rev. Cell Dev. Biol. 20, 759–779 (2004).
Faulkner, N. E. et al. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nature Cell Biol. 2, 784–791 (2000).
Vallee, R. B., Tai, C. & Faulkner, N. E. LIS1: cellular function of a disease-causing gene. Trends Cell Biol. 11, 155–160 (2001).
Sasaki, S. et al. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron 28, 681–696 (2000).
Niethammer, M. et al. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron 28, 697–711 (2000).
Sapir, T., Elbaum, M. & Reiner, O. Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit. EMBO J. 16, 6977–6984 (1997).
Reiner, O. et al. Isolation of a Miller–Dieker lissencephaly gene containing G protein β-subunit-like repeats. Nature 364, 717–721 (1993).
Pilz, D. T. et al. LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. Hum. Mol. Genet. 7, 2029–2037 (1998).
Sheeman, B. et al. Determinants of S. cerevisiae dynein localization and activation: implications for the mechanism of spindle positioning. Curr. Biol. 13, 364–372 (2003).
Lee, W. L., Oberle, J. R. & Cooper, J. A. The role of the lissencephaly protein Pac1 during nuclear migration in budding yeast. J. Cell Biol. 160, 355–364 (2003).
Xiang, X., Osmani, A. H., Osmani, S. A., Xin, M. & Morris, N. R. NudF, a nuclear migration gene in Aspergillus nidulans, is similar to the human LIS-1 gene required for neuronal migration. Mol. Biol. Cell 6, 297–310 (1995).
Efimov, V. P. & Morris, N. R. The LIS1-related NUDF protein of Aspergillus nidulans interacts with the coiled-coil domain of the NUDE/RO11 protein. J. Cell Biol. 150, 681–688 (2000).
Feng, Y. et al. LIS1 regulates CNS lamination by interacting with mNudE, a central component of the centrosome. Neuron 28, 665–679 (2000).
Sweeney, K. J., Prokscha, A. & Eichele, G. NudE-L, a novel Lis1-interacting protein, belongs to a family of vertebrate coiled-coil proteins. Mech. Dev. 101, 21–33 (2001).
Feng, Y. & Walsh, C. A. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron 44, 279–293 (2004).
Shu, T. et al. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron 44, 263–277 (2004).
Lee, W. -L., Kaiser, M. & Cooper, J. A. The offloading model for dynein function: differential function of motor subunits. J. Cell Biol. 168, 201–207 (2005).
Tarricone, C. et al. Coupling PAF signaling to dynein regulation; structure of LIS1 in complex with PAF-acetylhydrolase. Neuron 44, 809–821 (2004).
Adames, N. R. & Cooper, J. A. Microtubule interactions with the cell cortex causing nuclear movements in Saccharomyces cerevisiae. J. Cell Biol. 149, 863–874 (2000).
Carvalho, P., Gupta, M. L. Jr, Hoyt, M. A. & Pellman, D. Cell cycle control of kinesin-mediated transport of Bik1 (CLIP-170) regulates microtubule stability and dynein activation. Dev. Cell 6, 815–829 (2004).
Efimov, V. P. Roles of NUDE and NUDF proteins of Aspergillus nidulans: insights from intracellular localization and overexpression effects. Mol. Biol. Cell 14, 871–888 (2003).
Gomes, E. R., Jani, S. & Gundersen, G. G. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451–463 (2005).
Kaiser, C., Michaelis, S. & Mitchell, A. Methods in Yeast Genetics (Cold Spring Harbor Laboratory Press, Plainview, 1994).
Baudin, A., Ozierkalogeropoulos, O., Denouel, A., Lacroute, F. & Cullin, C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 21, 3329–3330 (1993).
Acknowledgements
We are grateful to R. Heil-Chapdelaine and S. Nelson for their advice and assistance. This work was supported by the American Heart Association Fellowship AHA40390 to J.L., Damon Runyon Cancer Research Foundation Fellowship DRG -1671 to W.-L.L., and NIH GM47337 to J.A.C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figure S1, movie legend and supplementary tables S1 and S2 (PDF 450 kb)
Supplementary Information
Supplementary movie S1a (AVI 149 kb)
Supplementary Information
Supplementary movie S1b (AVI 153 kb)
Rights and permissions
About this article
Cite this article
Li, J., Lee, WL. & Cooper, J. NudEL targets dynein to microtubule ends through LIS1. Nat Cell Biol 7, 686–690 (2005). https://doi.org/10.1038/ncb1273
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1273
This article is cited by
-
Pac1/LIS1 stabilizes an uninhibited conformation of dynein to coordinate its localization and activity
Nature Cell Biology (2020)
-
Genome Wide Analysis of WD40 Proteins in Saccharomyces cerevisiae and Their Orthologs in Candida albicans
The Protein Journal (2019)
-
She1 affects dynein through direct interactions with the microtubule and the dynein microtubule-binding domain
Nature Communications (2017)
-
Cytoplasmic dynein and early endosome transport
Cellular and Molecular Life Sciences (2015)
-
Connecting up and clearing out: how kinetochore attachment silences the spindle assembly checkpoint
Chromosoma (2012)