Abstract
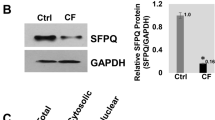
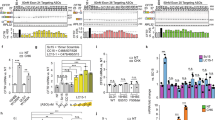
Spliceosome-mediated RNA trans-splicing (SMaRT) was investigated as a means for functionally correcting endogenous ΔF508 cystic fibrosis transmembrane conductance regulator (CFTR) transcripts using in vitro human cystic fibrosis (CF) polarized airway epithelia and in vivo human CF bronchial xenografts. Recombinant adenovirus (Ad.CFTR-PTM) encoding a pre-therapeutic molecule (PTM) targeted to CFTR intron 9 corrected transepithelial cyclic AMP (cAMP)-sensitive short-circuit current (Isc) in ΔF508 homozygous epithelia to a level 16% of that observed in normal human bronchial epithelia. Molecular analyses using RT-PCR and western blotting confirmed SMaRT-mediated partial correction of endogenous ΔF508 messenger RNA (mRNA) transcripts and protein. In an in vivo model of ΔF508 CF airway epithelia, human CF bronchial xenografts infected with Ad.CFTR-PTM also demonstrated partial correction of CFTR-mediated Cl− permeability at a level 22% of that seen in non-CF xenografts. These results provide functional evidence for SMaRT-mediated repair of mutant endogenous CFTR mRNA in intact polarized CF airway epithelial models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Korst, R.J. et al. Gene therapy for the respiratory manifestations of cystic fibrosis. Am. J. Respir. Crit. Care. Med. 151, S75–87 (1995).
Wagner, J.A., Chao, A.C. & Gardner, P. Molecular strategies for therapy of cystic fibrosis. Annu. Rev. Pharmacol. Toxicol. 35, 257–276 (1995).
Jiang, Q. & Engelhardt, J.F. Cellular heterogeneity of CFTR expression and function in the lung: implications for gene therapy of cystic fibrosis. Eur. J. Hum. Gen. 6, 12–31 (1998).
Imler, J.L. et al. Targeting cell-specific gene expression with an adenovirus vector containing the lacZ gene under the control of the CFTR promoter. Gene Ther. 3, 49–58 (1996).
Reed, R. Mechanisms of fidelity in pre-mRNA splicing. Curr. Opin. Cell Biol. 12, 340–345 (2000).
Eul, J., Graessmann, M. & Graessmann, A. Experimental evidence for RNA trans-splicing in mammalian cells. EMBO J. 14, 3226–3235 (1995).
Caudevilla, C. et al. Natural trans-splicing in carnitine octanoyltransferase pre-mRNAs in rat liver. Proc. Natl. Acad. Sci. USA 95, 12185–12190 (1998).
Mansfield, S.G. et al. Repair of CFTR mRNA by spliceosome-mediated RNA trans-splicing. Gene Ther. 7, 1885–1895 (2000).
Puttaraju, M., Jamison, S.F., Mansfield, S.G., Garcia-Blanco, M.A. & Mitchell, L.G. Spliceosome-mediated RNA trans-splicing as a tool for gene therapy. Nat. Biotechnol. 17, 246–252 (1999).
Dallinger, G. et al. Collagen 17A1 gene correction using spliceosome mediated RNA trans-splicing (SMaRT™) technology. J. Invest. Dermatol. 115, 332 (2000).
Engelhardt, J.F. et al. Direct gene transfer of human CFTR into human bronchial epithelia of xenografts with E1-deleted adenoviruses. Nat. Genet. 4, 27–34 (1993).
Puttaraju, M., DiPasquale, J., Baker, C.C., Mitchell, L.G. & Garcia-Blanco, M.A. Messenger RNA repair and restoration of protein function by spliceosome- mediated RNA trans-splicing. Mol. Ther. 4, 105–114 (2001).
Zhang, Y., Yankaskas, J., Wilson, J. & Engelhardt, J.F. In vivo analysis of fluid transport in cystic fibrosis airway epithelia of bronchial xenografts. Am. J. Physiol. 270, C1326–1335 (1996).
Zhang, Y., Jiang, Q., Dudus, L., Yankaskas, J.R. & Engelhardt, J.F. Vector-specific complementation profiles of two independent primary defects in cystic fibrosis airways. Hum. Gene Ther. 9, 635–648 (1998).
Wei, L.Y., Stutts, M.J., Hoffman, M.M. & Roepe, P.D. Overexpression of the cystic fibrosis transmembrane conductance regulator in NIH 3T3 cells lowers membrane potential and intracellular pH and confers a multidrug resistance phenotype. Biophys. J. 69, 883–895 (1995).
Schiavi, S.C. et al. Biosynthetic and growth abnormalities are associated with high-level expression of CFTR in heterologous cells. Am. J. Physiol. 270, C341–351 (1996).
Mohammad-Panah, R. et al. Hyperexpression of recombinant CFTR in heterologous cells alters its physiological properties. Am. J. Physiol. 274, C310–318 (1998).
Chu, C.S., Trapnell, B.C., Curristin, S.M., Cutting, G.R. & Crystal, R.G. Extensive posttranscriptional deletion of the coding sequences for part of nucleotide-binding fold 1 in respiratory epithelial mRNA transcripts of the cystic fibrosis transmembrane conductance regulator gene is not associated with the clinical manifestations of cystic fibrosis. J. Clin. Invest. 90, 785–790 (1992).
Trapnell, B.C. et al. Expression of the cystic fibrosis transmembrane conductance regulator gene in the respiratory tract of normal individuals and individuals with cystic fibrosis. Proc. Natl. Acad. Sci. USA 88, 6565–6569 (1991).
Johnson, L.G. et al. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 2, 21–25 (1992).
Engelhardt, J.F. et al. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat. Genet. 2, 240–248 (1992).
Engelhardt, J.F., Zepeda, M., Cohn, J.A., Yankaskas, J.R. & Wilson, J.M. Expression of the cystic fibrosis gene in adult human lung. J. Clin. Invest. 93, 737–749 (1994).
Cotton, C.U., Stutts, M.J., Knowles, M.R., Gatzy, J.T. & Boucher, R.C. Abnormal apical cell membrane in cystic fibrosis respiratory epithelium. An in vitro electrophysiologic analysis. J. Clin. Invest. 79, 80–85 (1987).
Kunzelmann, K., Kathofer, S. & Greger, R. Na+ and Cl− conductances in airway epithelial cells: increased Na+ conductance in cystic fibrosis. Pflugers. Arch. 431, 1–9 (1995).
Wilson, J.M. Adenoviral vectors. In Current protocols in human genetics. (eds Dracopoli, N.C. et al.) 12.4.1–12.4.18 (John Wiley & Sons, Inc., New York; 1998).
Anderson, R.D., Haskell, R.E., Xia, H., Roessler, B.J. & Davidson, B.L. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 7, 1034–1038 (2000).
Duan, D., Zhang, Y. & Engelhardt, J.F. Gene delivery to the airway. In Current protocols in human genetics. (eds Dracopoli, N.C. et al.) 13.9.1–13.9.34 (John Wiley & Sons, Inc., New York; 1998).
Zabner, J., Smith, J.J., Karp, P.H., Widdicombe, J.H. & Welsh, M.J. Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol. Cell. 2, 397–403 (1998).
Acknowledgements
This work was supported by SBIR grant R44 DK56526 from the National Institutes of Health (L.G.M.) and a grant from the Cystic Fibrosis Foundation (S.G.M.) to Intronn, and by the Iowa Center for Gene Therapy of Cystic Fibrosis and Other Genetic Diseases co-funded by the National Institutes of Health and the Cystic Fibrosis Foundation (P30 DK54759; J.F.E.). The corresponding author has no financial interests in Intronn Inc. and does not consult for this company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Jiang, Q., Mansfield, S. et al. Partial correction of endogenous ΔF508 CFTR in human cystic fibrosis airway epithelia by spliceosome-mediated RNA trans-splicing. Nat Biotechnol 20, 47–52 (2002). https://doi.org/10.1038/nbt0102-47
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt0102-47
This article is cited by
-
Construction and validation of an RNA trans-splicing molecule suitable to repair a large number of COL7A1 mutations
Gene Therapy (2016)
-
Trans-splicing repair of mutant p53 suppresses the growth of hepatocellular carcinoma cells in vitro and in vivo
Scientific Reports (2015)
-
Repair of Rhodopsin mRNA by Spliceosome-Mediated RNA Trans -Splicing: A New Approach for Autosomal Dominant Retinitis Pigmentosa
Molecular Therapy (2015)
-
Alternative splicing: a pivotal step between eukaryotic transcription and translation
Nature Reviews Molecular Cell Biology (2013)
-
Repair of Mybpc3 mRNA by 5′-trans-splicing in a Mouse Model of Hypertrophic Cardiomyopathy
Molecular Therapy - Nucleic Acids (2013)