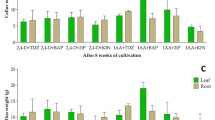
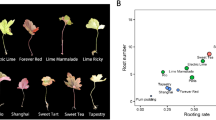
Abstract
A plethora of important, chemically diverse natural products are derived from plants1. In principle, plant cell culture offers an attractive option for producing many of these compounds2,3. However, it is often not commercially viable because of difficulties associated with culturing dedifferentiated plant cells (DDCs) on an industrial scale3. To bypass the dedifferentiation step, we isolated and cultured innately undifferentiated cambial meristematic cells (CMCs). Using a combination of deep sequencing technologies, we identified marker genes and transcriptional programs consistent with a stem cell identity. This notion was further supported by the morphology of CMCs, their hypersensitivity to γ-irradiation and radiomimetic drugs and their ability to differentiate at high frequency. Suspension culture of CMCs derived from Taxus cuspidata, the source of the key anticancer drug, paclitaxel (Taxol)2,3, circumvented obstacles routinely associated with the commercial growth of DDCs. These cells may provide a cost-effective and environmentally friendly platform for sustainable production of a variety of important plant natural products.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Schmidt, B.M., Ribnicky, D.M., Lipsky, P.E. & Raskin, I. Revisiting the ancient concept of botanical therapeutics. Nat. Chem. Biol. 3, 360–366 (2007).
Croteau, R., Ketchum, R.E.B., Long, R.M., Kaspera, R. & Wildung, M.R. Taxol biosynthesis and molecular genetics. Phytochem. Rev. 5, 75–97 (2006).
Roberts, S.C. Production and engineering of terpenoids in plant cell culture. Nat. Chem. Biol. 3, 387–395 (2007).
Laux, T. The stem cell concept in plants: a matter of debate. Cell 113, 281–283 (2003).
Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 37, 169–180 (2007).
Sugimoto, K., Jiao, Y. & Meyerowitz, E.M. Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 18, 463–471 (2010).
Grafi, G. et al. Histone methylation controls telomerase-independent telomere lengthening in cells undergoing dedifferentiation. Dev. Biol. 306, 838–846 (2007).
Baebler, S. et al. Establishment of cell suspension cultures of yew (Taxus x Media Rehd) and assessment of their genomic stability. In Vitro Cell. Dev. Biol. Plant 41, 338–343 (2005).
Ye, Z.-H. Vascular tissue differentiation and pattern formation in plants. Annu. Rev. Plant Biol. 53, 183–202 (2002).
Strobel, G.A. et al. Taxol formation in yew-Taxus. Plant Sci. 92, 1–12 (1993).
Frankenstein, C., Eckstein, D. & Schmitt, U. The onset of cambium activity - A matter of agreement? Dendrochronologia 23, 57–62 (2005).
Moore, R., Clark, W.D., Stern, K.R. & Vodopich, D. (eds.) Botany (Wm.C. Brown, Dubuque, lowa, USA; 1995).
Turner, S., Gallois, P. & Brown, D. Tracheary element differentiation. Annu. Rev. Plant Biol. 58, 407–433 (2007).
Ito, Y. et al. Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313, 842–845 (2006).
Fulcher, N. & Sablowski, R. Hypersensitivity to DNA damage in plant stem cell niches. Proc. Natl. Acad. Sci. USA 106, 20984–20988 (2009).
Shendure, J. & Ji, H. Next-generation DNA sequencing. Nat. Biotechnol. 26, 1135–1145 (2008).
Fisher, K. & Turner, S. PXY, a receptor-like kinase essential for maintaining polarity during plant vascular-tissue development. Curr. Biol. 17, 1061–1066 (2007).
Mähönen, A.P. et al. A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes Dev. 14, 2938–2943 (2000).
Nieminen, K. et al. Cytokinin signaling regulates cambial development in popular. Proc. Natl. Acad. Sci. USA 105, 20032–20037 (2008).
Rando, T.A. The immortal strand hypothesis: segregation and reconstruction. Cell 129, 1239–1243 (2007).
Joshi, J.B., Elias, C.B. & Patole, M.S. Role of hydrodynamic shear in the cultivation of animal, plant and microbial cells. Chem. Eng. J. 62, 121–141 (1996).
Wang, C., Wu, J. & Mei, X. Enhanced taxol production and release in Taxus chinesis cell suspension cultures with selected organic solvents and sucrose feeding. Biotechnol. Prog. 17, 89–94 (2001).
Mirjalili, N. & Linden, J.C. Methyl jasmonate induced production of Taxol in suspension cultures of Taxus cuspidata: Ethylene interaction and induction models. Biotechnol. Prog. 12, 110–118 (1996).
Wu, Z.L., Yuan, Y.-J., Ma, Z.-H. & Hu, Z.D. Kinetics of two-liquid-phase Taxus cuspidata cell culture for production of Taxol. Biochem. Eng. J. 5, 137–142 (2000).
Yang, S.-J., Fang, J.-M. & Cheng, Y.-S. Lignans, flavonoids and phenolic derivatives from Taxus mairei. J. Chinese Chem. Soc. 46, 811–818 (1999).
Bai, J. et al. Production of biologically active taxoids by a callus culture of Taxus cuspidata. J. Nat. Prod. 67, 58–63 (2004).
Leung, K.W. & Wong, A.S.-T. Pharmacology of ginsenosides: a literature review. Chin. Med. 5, 20 (2010).
Dan, M. et al. Metabolite profiling of Panax notoginseng using UPLC-ESI-MS. Phytochemistry 69, 2237–2244 (2008).
Reynolds, L.B. Effects of harvest date on some chemical and physical characteristics of American ginseng (Panax quinquefolius L.). J. Herbs Spices Med. Plants 6, 63–69 (1998).
Kutchan, T. & Dixon, R.A. Physiology and metabolism: Secondary metabolism: nature's chemical reservoir under deconvolution. Curr. Opin. Plant Biol. 8, 227–229 (2005).
Gamborg, O.L., Miller, R.A. & Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50, 151–158 (1968).
Zang, X., Mei, X.-G., Zhang, C.-H., Lu, C.T. & Ke, T. Improved paclitaxel accumulation in cell suspension cultures of Taxus chinensis by brassinolide. Biotechnol. Lett. 23, 1047–1049 (2001).
Yukimune, Y., Tabata, H., Higashi, Y. & Hara, Y. Methyl jasmonate-induced overproduction of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat. Biotechnol. 14, 1129–1132 (1996).
Fulcher, N. & Sablowski, R. Hypersensitivity to DNA damage in plant stem cell niches. Proc. Natl. Acad. Sci. USA 106, 20984–20988 (2009).
Zhu, Y.Y., Machleder, E.M., Chenchik, A., Li, R. & Siebert, P.D. Reverse transcriptase template switching: A SMARTTM approach for full-length cDNA library construction. BioTech. 30, 892–897 (2001).
Zhulidov, P.A. et al. Simple cDNA normalization using Kamchatka crab duplex-specific nuclease. Nucleic Acids Res. 32, e37 (2004).
Margulies, M. et al. Genome sequencing in open microfabricated high-density picoliter reactors. Nature 437, 376–380 (2005).
Brenner, S. et al. Gene expression analysis by massively parallel signature sequencing (MPSS) on microbead arrays. Nat. Biotechnol. 18, 630–634 (2000).
Schmid, R. & Blaxter, M.L. annot8r: GO, EC and KEGG annotation of EST datasets. BMC Bioinformatics 9, 180 (2008).
Robinson, M.D., McCarthy, D.J. & Smyth, G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Gentleman, R.C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004).
Bullard, J.H., Purdom, E., Hansen, K.D. & Dudoit, S. Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinformatics 11, 94 (2010).
Nolan, T., Hands, R.E. & Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 1, 1559–1582 (2006).
Kataoka, T. et al. Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. Plant Cell 16, 2693–2704 (2004).
Lee, Y. et al. The Arabidopsis phosphatidylinositol 3-kinase is important for pollen development 1. Plant Physiol. 147, 1886–1897 (2008).
Acknowledgements
T.W. was awarded a BBSRC CASE PhD studentship. This project was funded in part by a grant from the Korea Institute for Advancement of Technology (KIAT) (R & D project number: 10030175), the Ministry of Knowledge Economy (MKE), Republic of Korea to E.-K.L., J.H.P., S.M.H. and G.J.L. R.A. was supported by a scholarship from HEC Pakistan. E.K. was supported by a studentship from the Engineering and Physical Sciences Research Council. We acknowledge the expert technical assistance of A. Montazam and D. Cleven for Roche 454 sequencing and M. Thomson for Illumina Solexa sequencing. Further, S. Bridgett and U. Trivedi provided invaluable input for bioinformatic analysis of the deep sequencing data. All sequencing was undertaken at the GenePool facility, University of Edinburgh.
Author information
Authors and Affiliations
Contributions
E.-K.L., Y.-W.J., J.H.P., T.W. and B.-W.Y. performed experiments. R.A., E.K., S.T. and F.H. contributed to bioinformatic and statistical analysis. Z.Y., Y.M.Y. and S.M.H. carried out experiments. A.E. co-supervised E.K. E.-K.L., Y.-W.J. and G.J.L. formulated experiments. G.J.L. and E.-K.L. wrote the paper. All authors discussed results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
E.K.L. and Y.W.J. hold stock in Unhwa Corp.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–7 and Supplementary Figs. 1–17 (PDF 2149 kb)
Supplementary Data Set 1
Assembled T. cuspidata transcriptome. (PDF 27516 kb)
Supplementary Data Set 2
BLAST analysis of T. cuspidata contigs. (PDF 1663 kb)
Supplementary Data Set 3
Digital gene expression tag profiling of CMCs. (PDF 1482 kb)
Supplementary Data Set 4
Differentially expressed contigs between T. cuspidata CMCs and DDCs. (PDF 106 kb)
Supplementary Fig. 7
Comparison of the cultural properties of the given cell lines in a 3 L air-lift bioreactor. (PDF 1566 kb)
Rights and permissions
About this article
Cite this article
Lee, EK., Jin, YW., Park, J. et al. Cultured cambial meristematic cells as a source of plant natural products. Nat Biotechnol 28, 1213–1217 (2010). https://doi.org/10.1038/nbt.1693
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.1693
This article is cited by
-
Suspension culture of stem cells established of Calendula officinalis L.
Scientific Reports (2024)
-
Isolation, culture of protoplasts of Angelica gigas Nakai and regeneration of plants via somatic embryogenesis
Plant Cell, Tissue and Organ Culture (PCTOC) (2024)
-
Omics analyses of Rehmannia glutinosa dedifferentiated and cambial meristematic cells reveal mechanisms of catalpol and indole alkaloid biosynthesis
BMC Plant Biology (2023)
-
Increased paclitaxel recovery from Taxus baccata vascular stem cells using novel in situ product recovery approaches
Bioresources and Bioprocessing (2023)
-
An Overview on Taxol Production Technology and Its Applications as Anticancer Agent
Biotechnology and Bioprocess Engineering (2022)