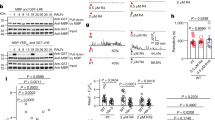
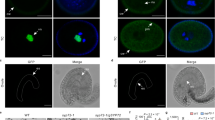
Abstract
Glutamate receptors are well characterized channels that mediate cell-to-cell communication during neurotransmission in animals, but their functional role in organisms without a nervous system remains unclear. In plants, genes of the GLUTAMATE RECEPTOR-LIKE (GLR) family have been implicated in defence against pathogens, reproduction, control of stomata aperture and light signal transduction1,2,3,4,5. However, the large number of GLR genes present in angiosperm genomes (20 to 70)6 has prevented the observation of strong phenotypes in loss-of-function mutants. Here we show that in the basal land plant Physcomitrella patens, mutation of the GLR genes GLR1 and GLR2 causes failure of sperm cells to target the female reproductive organs. In addition, we show that GLR genes encode non-selective Ca2+-permeable channels that can regulate cytoplasmic Ca2+ and are needed to induce the expression of a BELL1-like transcription factor essential for zygote development. Our work reveals functions for GLR channels in sperm chemotaxis and transcriptional regulation. Sperm chemotaxis is essential for fertilization in both animals and early land plants such as bryophytes and pteridophytes. Therefore, our results suggest that ionotropic glutamate receptors may have been conserved throughout plant evolution to mediate cell-to-cell communication during sexual reproduction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Forde, B. G. & Roberts, M. R. Glutamate receptor-like channels in plants: a role as amino acid sensors in plant defence? F1000Prime Rep. 6, 37 (2014)
Cho, D. et al. De-regulated expression of the plant glutamate receptor homolog AtGLR3.1 impairs long-term Ca2+-programmed stomatal closure. Plant J. 58, 437–449 (2009)
Lam, H. M. et al. Glutamate-receptor genes in plants. Nature 396, 125–126 (1998)
Michard, E. et al. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil d-serine. Science 332, 434–437 (2011)
Mousavi, S. A., Chauvin, A., Pascaud, F., Kellenberger, S. & Farmer, E. E. GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500, 422–426 (2013)
Chiu, J. C. et al. Phylogenetic and expression analysis of the glutamate-receptor-like gene family in Arabidopsis thaliana. Mol. Biol. Evol. 19, 1066–1082 (2002)
Verret, F., Wheeler, G., Taylor, A. R., Farnham, G. & Brownlee, C. Calcium channels in photosynthetic eukaryotes: implications for evolution of calcium-based signalling. New Phytol. 187, 23–43 (2010)
De Bortoli, S., Teardo, E., Szabò, I., Morosinotto, T. & Alboresi, A. Evolutionary insight into the ionotropic glutamate receptor superfamily of photosynthetic organisms. Biophys. Chem. 218, 14–26 (2016)
Ortiz-Ramírez, C. et al. A transcriptome atlas of Physcomitrella patens provides insights into the evolution and development of land plants. Mol. Plant 9, 205–220 (2016)
Perroud, P. F., Cove, D. J., Quatrano, R. S. & McDaniel, S. F. An experimental method to facilitate the identification of hybrid sporophytes in the moss Physcomitrella patens using fluorescent tagged lines. New Phytol. 191, 301–306 (2011)
Ren, D. et al. A sperm ion channel required for sperm motility and male fertility. Nature 413, 603–609 (2001)
Lilienbaum, A. & Israël, A. From calcium to NF-κB signaling pathways in neurons. Mol. Cell. Biol. 23, 2680–2698 (2003)
Hogan, P. G., Chen, L., Nardone, J. & Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 17, 2205–2232 (2003)
Rutjens, B. et al. Shoot apical meristem function in Arabidopsis requires the combined activities of three BEL1-like homeodomain proteins. Plant J. 58, 641–654 (2009)
Reiser, L. et al. The BELL1 gene encodes a homeodomain protein involved in pattern formation in the Arabidopsis ovule primordium. Cell 83, 735–742 (1995)
Brambilla, V. et al. Genetic and molecular interactions between BELL1 and MADS box factors support ovule development in Arabidopsis. Plant Cell 19, 2544–2556 (2007)
Bowman, J. L., Sakakibara, K., Furumizu, C. & Dierschke, T. Evolution in the cycles of life. Annu. Rev. Genet. 50, 133–154 (2016)
Horst, N. A. et al. A single homeobox gene triggers phase transition, embryogenesis and asexual reproduction. Nat. Plants 2, 15209 (2016)
Lee, J. H., Lin, H., Joo, S. & Goodenough, U. Early sexual origins of homeoprotein heterodimerization and evolution of the plant KNOX/BELL family. Cell 133, 829–840 (2008)
Mutwil, M. et al. PlaNet: combined sequence and expression comparisons across plant networks derived from seven species. Plant Cell 23, 895–910 (2011)
Borges, F. et al. Comparative transcriptomics of Arabidopsis sperm cells. Plant Physiol. 148, 1168–1181 (2008)
Hu, J. H. et al. Identification of glutamate receptors and transporters in mouse and human sperm. J. Androl. 25, 140–146 (2004)
Ashton, N. & Cove, D. The isolation and preliminary characterisation of auxotrophic and analogue resistant mutants of the moss, Physcomitreila patens. Mol. Genet. Genomics 154, 87–95 (1977)
Reski, R. & Abel, W. O. Induction of budding on chloronemata and caulonemata of the moss, Physcomitrella patens, using isopentenyladenine. Planta 165, 354–358 (1985)
Hohe, A., Rensing, S. A., Mildner, M., Lang, D. & Reski, R. Day length and temperature strongly influence sexual reproduction and expression of a novel MADS-box gene in the moss Physcomitrella patens. Plant Biol. 4, 595–602 (2002)
Reyes, R. et al. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J. Biol. Chem. 273, 30863–30869 (1998)
Jurman, M. E., Boland, L. M., Liu, Y. & Yellen, G. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques 17, 876–881 (1994)
Li, C. & Wong, W. H. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc. Natl Acad. Sci. USA 98, 31–36 (2001)
Damineli, D. S. C., Portes, M. T. & Feijó, J. A. Oscillatory signatures underlie growth regimes in Arabidopsis pollen tubes: computational methods to estimate tip location, periodicity, and synchronization in growing cells. J. Exp. Bot. 12, 3267–3281 (2017)
Huang, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protocols 4, 44–57 (2009)
Acknowledgements
We thank P. F. Perroud for the Gransden GFP and Villersexel RFP lines; L. Dolan and T. Tan for the pBNRF, pBHrev and p108GW35S vectors; M. Hasebe for the pTN83 construct; S. Rensing for providing us access to the Nimblegen_Ppat_SR_-exp_HX12 array and the Institute for Research in Biomedicine (IRB)/Functional Genomics Core (FGC) (Barcelona) for microarray processing; J. Kudla for the pEF1-YC3.6 vector. C.O.R. and M.H.C. acknowledge fellowships from MarieCurie ITN-Plant Origins (FP7-PEOPLE-ITN-2008). J.A.F. acknowledges grants from the Fundação para a Ciêncua e Tecnologia (FCT)-Portugal (BEX-BCM/0376/2012; PTDC/BIA-PLA/4018/2012) and the National Science Foundation (NSF)-US (MCB 1616437/2016).
Author information
Authors and Affiliations
Contributions
C.O.R. was involved in generating all experimental data except electrophysiology. E.M. and A.A.S. performed the electrophysiology. D.S.C.D. performed image and data analysis. M.H.C. and J.D.B. isolated sperm cells, purified RNA and analysed transcriptomic data. J.A.F. conceived the project. J.A.F. and C.O.R. wrote the manuscript and conceived all experiments. All authors discussed and improved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks J. Kudla and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Measurement of caulonema filaments and cross-fertilization.
a, Growth of protonema colonies under different Ca2+ concentrations. The initial colony diameter was subtracted from the diameter at day 5. Data are from 3 biological replicates and several independent colony measurements. n = 59 (WT), n = 58 (glr1), n = 58 (glr2) n = 55 (glr1/2). Error bars denote s.d. There was a statistically significant difference between Ca2+ treatments (P < 0.001, F156.3, two-way ANOVA) but not between genotypes (P = 0.952, two-way ANOVA). Colony diameter in samples treated with 100 and 1,000 μM Ca2+ was significantly different from untreated samples. *P = 0.01, **P < 0.001, Holm–Sidak multiple comparison test. b, Caulonema cells (white arrows) from wild-type lines and the glr1/2 mutant have similar morphologies. Scale bars, 500 μM. Images are representative of 13 images. c, Caulonema length from filaments induced in darkness. There was no significant difference between the wild type (n = 148) and glr1/2 mutant (n = 180). Error bars represent s.d. Results were obtained from 3 biological replicates. d, Self-fertilization events (top) and outcrossing events (bottom) were identified in cross-fertilization experiments between Vsx RFP (paternal line) and glr1/2 (maternal line). Scale bars, 200 μm. Images are representative of 4 images. e, Sporophyte production rates in cross-fertilization experiments using different genotypes are shown. Sporophyte production in glr1/2 was as high as in the wild type (outcrossing rates), suggesting full phenotypic complementation when Vsx sperm cells are provided. 50 gametophores per replicate were analysed. Data are mean ± s.d. from 4 biological replicates.
Extended Data Figure 2 Display of an isolated reproductive unit, and quantification of cytosolic calcium and sporophyte production.
a, An intact open archegonium, several antheridia, and a sperm cell cluster are shown (arrows). In most samples, only one antheridium was ready to release the sperm cells at the time of the experiment. The antheridium from which the sperm cell cluster was released is indicated by an asterisk. After 1 min, the released sperm cells became fully motile and individual cells began to separate from the protein matrix (arrowheads). Scale bar, 30 μm. Image is representative of 6 images. b, Basal cytosolic calcium in control cells (transfected with pCI and pEF1-YC3.6) and cells co-expressing GLR1 and YC3.6 exposed to medium with no calcium. n = 29 (control), n = 15 (GLR1); biological replicates. There was a statistically significant difference between the two samples. *P = 3 × 10−8, Welch’s t-test for unequal variances. Violin plots show curves of density estimates, boxplots and white points with lines are mean and s.e.m. c, The GLR2::BELL1 complementation line shows a similar sporophyte production rate to the glr1/2 mutant line. The percentage of gametophores with sporophytes from the total number of gametophores is shown. Data are mean ± s.d. from several independent biological replicates. n = 12 (WT), n = 7 (glr1/2), n = 4 (GLR2::BELL1). 100 gametophores were counted per sample. Box plot lines show outliers.
Extended Data Figure 3 Gene network analysis for GLR1 and GLR2.
a, GLR2 co-expression gene network showing genes one and two steps away from the query gene. Gene Ontology (GO) analysis of the 151 genes that integrate the network shows only two enriched categories: transcriptional regulation and protein kinase activity (modified Fisher exact P value). The gene families represented in the two clusters are shown in italics. Highlighted nodes represent transcription factors genes interacting with GLR2 in the network. b, GLR1 co-expression gene network showing genes two steps away from the query gene. No enriched Gene Ontology categories were found. GLR1 is highlighted at the centre of the network. Shapes represent label co-occurrences used to group genes according to Pfam domains and PLAZA families. See Supplementary Table 3 for the complete gene lists and annotations.
Extended Data Figure 4 Effect of Gd3+ and AP5 on 35S::PpGLR1 protoplasts currents.
a, Typical currents recorded in protoplasts from 35S::PpGLR1 protonema under the whole-cell configuration before and after the application of 0.5 and 1 mM Gd3+. b, Standardized current–voltage curves of stationary currents recorded in experiments as shown in a. n = 3 for all conditions. c, Effect of AP5 (200 μM) on typical currents recorded in protoplasts from 35S::PpGLR1 (n = 5). d, Standardized currents corresponding to c, recorded before (black circles) and after (red circles) the addition of 200 μM AP5 (n = 4). In all cases n represents biological replicates. Error bars denote s.e.m.
Extended Data Figure 5 Effect of Gd3+, CNQX and AP5 on COS-7 cells expressing GLR1.
a, Typical currents recorded in COS-7 cells transfected with pCI-PpGLR1 before and after the addition of 1 mM Gd3+. b, Standardized current–voltage curves of experiments in a. Currents were recorded in control conditions before Gd3+ application (black circles), and after the addition of 1 mM Gd3+ (red circles) (n = 3 for control and treatment). c, Typical currents recorded in transfected COS-7 cells expressing GLR1 in control solution (left) and after the addition of 50 μM CNQX (right). d, Standardized current–voltage curves of experiments in c. Currents were recorded in control conditions (black circles), and after the addition of 50 μM CNQX (red circles) (n = 3). e, Typical currents recorded in COS-7 cells transfected with pCI-PpGLR1 before and after the addition of AP5 (200 μM). f, Standardized current–voltage curves of experiments presented in e. Currents were recorded in control conditions (black circles) and after the addition of 200 μM AP5 (red circles) (n = 4). Standard solutions are identical to Fig. 4e, f. Pipette contained 146 mM Cl− and 140 mM Na+, bath solution contained 150 mM Cl−, 5 mM Ca2+ and 10 mM Na+ as charge carriers. Error bars denote s.e.m. In all cases n represents biological replicates.
Extended Data Figure 6 GLR1 is a non-selective channel.
a, Typical currents recorded in wild-type protoplasts from protonema with 10 or 110 mM Na+. b, Standardized current–voltage curves of experiments in a. 10 mM Na+ (black circles), 110 mM Na+ (red circles) (n = 4). c, Typical currents recorded in COS-7 cells transfected with pCI-PpGLR1 with external solutions containing: (i) 10 mM Na+, 5 mM Ca2+, 150 mM Cl−; (ii) 140 mM Na+, 5 mM Ca2+, 150 mM Cl−; or (iii) 10 mM Na+, 70 mM Ca2+, 150 mM Cl−. d, Standardized current–voltage curves of experiments presented in c (n = 3). e, Representative current traces from COS-7 cells transfected with pCI-PpGLR1 in an external solution containing 10 mM Ca2+ and 10 mM Cl− without Na+ (left) and with 130 mM Na-gluconate (right) applied by perfusion. Pipette solutions were as in Fig. 4g, h. f, Standardized current–voltage curves of experiments shown in e. Na+-free solutions (red circles), 130 mM Na+ (black circles) (n = 3). When the external Na+ concentration increased from 10 to 140 mM, a current increase was observed, demonstrating the permeability of GLR1 for Na+ shown in c and d. Furthermore, Na+ permeability was confirmed as the current intensity increased and the reversal potential also significantly shifted to the right when adding Na+ to the external solution, as shown in e and f. g, Representative current traces from control cells (left) and COS-7 cells transfected with pCI-PpGLR1 (right) in 70 mM Ca2+ bathing solution (experimental conditions as in Fig. 4g, h). h, Current–voltage curve depicting average stationary current intensity as shown in g. Control (red circles) and pCI-PpGLR1(black circles) (n = 3). The increase in current by external Ca2+ demonstrates a permeability of GLR1 to Ca2+. Together with data presented in c and d, these results suggest a complex effect of external Ca2+. Indeed, while g and h demonstrate a Ca2+ permeation in conditions in which no Na+ and low (6 mM) Cl− are present, c and d show a decrease of ionic currents by high extracellular Ca2+ when the bath contains 10 mM Na+ and 150 mM Cl−. Panels e–h also suggest that Cl− may pass through the channel accounting for the portion of outward current. Error bars denote s.e.m. In all cases n represents biological replicates.
Extended Data Figure 7 Schematic representation of the constructs used for the generation of glr1 and glr1/2 mutants by homologous recombination.
a, GLR1 endogenous genomic locus (top) and pBNRF plasmid used for transformation (lines) are shown. The boxes and lines between the boxes represent exons and introns, respectively. Grey boxes represent untranslated regions (UTRs) of the gene, and small arrows indicate the approximate position where primers bind to the DNA sequence. Broken lines denote the flanking genomic regions of the genes used for homologous recombination. b, The endogenous genomic locus of GLR2 (top) and the pBHrev construct used for transformation (bottom). c, PCR showing deletion of the GLR1 gene from glr1 knockout mutants and the correct insertion of the construct used for transformation. d, Same as c but for the glr1/2 double-knockout mutants. See Supplementary Information for uncropped gel images.
Extended Data Figure 8 Independent mutant lines have similar phenotypes.
a, All glr1 mutant lines show a significant difference in sporophyte production when compared to wild-type lines. P ≤ 0.012, two-tailed t-test. 100 gametophores were counted per sample (biological replicates); n = 12 (WT), n = 8 (glr1-19), n = 4 (glr1-8), n = 3 (glr1-14). b, Similarly, all glr2 mutant lines produced very few sporophytes compared to wild-type lines. P ≤ 0.004, Mann–Whitney U-test. Data from several biological replicates; n = 12 (WT), n = 9 (glr1/2-63), n = 7 (glr1/2-11), n = 4 (glr1/2-16). 100 gametophores were counted per sample. c, All complementation lines had significantly bigger spores than spores of glr1/2 double-knockout lines. P ≤ 0.001, Mann–Whitney U-test. Spores from 3 biological replicates were pooled and measured. Data points represent individually measured spores. n = 82 (WT), n = 174 (glr1/2) n = 117 (GLR2::BELL1-57), n = 284 (GLR2::BELL1-18), n = 202 (GLR2::BELL1-10). d, Similarly, GLR2::BELL1 sporophytes clearly produced more spores than glr1/2 mutants (P ≤ 0.022, two-tailed t-test). Data from several biological replicates. n = 9 (WT), n = 6 (GLR2::BELL1-18, GLR2::BELL1-10, glr1/2), n = 3 (GLR2::BELL1-57). Box plots show 25th and 75th percentiles, and lines indicate outliers.
Extended Data Figure 9 Schematic representation of the constructs used for the generation of glr2 and GLR2::BELL1 mutants by homologous recombination.
a, GLR2 endogenous genomic locus (top) and pTN83 plasmid used for transformation (bottom). The boxes and lines between the boxes represent exons and introns, respectively. Small arrows indicate the approximate position where primers bind to the DNA sequence. Broken lines denote the genomic regions used for homologous recombination. b, The endogenous genomic locus in the glr1/2 mutant (top), and pZB1 construct used for transformation (bottom). c, PCR showing the deletion of the GLR2 gene from glr2 single-knockout mutants and the correct insertion of the construct used for transformation. d, Hygromycin could not be detected in GLR2::BELL1 complementation mutants and PCR analyses show correct insertion of the construct. e, RT–PCR experiments show that GLR1 transcripts are more abundant in 35S::PpGLR1 lines. 25 cycles of PCR amplification using cDNA obtained from protonema were used to amplify GLR1 in wild-type and overexpression lines. α-tubulin (TUB) was used as a control. See Supplementary Information for uncropped gel images.
Supplementary information
Supplementary Information
This file contains the uncropped gel images presented in Extended Data Figures 7 and 9. (PDF 1218 kb)
Supplementary Table 1
A list of primers used in this study. The sequence and code identifying each primer is shown. (XLSX 16 kb)
Supplementary Table 2
An extended list of up and down regulated genes in the double KO GLR mutant. (XLSX 5010 kb)
Supplementary Table 3
Genes integrating the PpGLR2 gene network. Genes and their functional annotation based on Arabidopsis homology located one and two steps away from the query gene are presented. (XLSX 18 kb)
wt sperm cell typical swimming behaviour under our conditions
Sperm cells maintained a relatively constant speed and always advance in a helicoidal manner. (MOV 1472 kb)
Ppglr1/2 sperm cells display very similar swimming behaviour compared to wt
Ppglr1/2 sperm cells display very similar swimming behaviour compared to wt. (MOV 3245 kb)
Typically at least one wt sperm cell per sample was able to reach the archegonia opening
Typically at least one wt sperm cell per sample was able to reach the archegonia opening. (MOV 3757 kb)
wt sperm cell entering the archegonia channel
wt sperm cell entering the archegonia channel (MOV 6221 kb)
Ppglr1/2 sperm cell failed to modify its trajectory and misses the archegonia opening
Ppglr1/2 sperm cell failed to modify its trajectory and misses the archegonia opening (MOV 5428 kb)
Some Ppglr1/2 sperm cells get close to the archegonia opening but change swimming direction
Some Ppglr1/2 sperm cells get close to the archegonia opening but change swimming direction (MOV 5271 kb)
Rights and permissions
About this article
Cite this article
Ortiz-Ramírez, C., Michard, E., Simon, A. et al. GLUTAMATE RECEPTOR-LIKE channels are essential for chemotaxis and reproduction in mosses. Nature 549, 91–95 (2017). https://doi.org/10.1038/nature23478
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature23478
This article is cited by
-
Genome-wide identification, expression profiling, and network analysis of calcium and cadmium transporters in rice (Oryza sativa L.)
Cereal Research Communications (2024)
-
Protocol: an improved method for inducing sporophyte generation in the model moss Physcomitrium patens under nitrogen starvation
Plant Methods (2023)
-
The origin of a land flora
Nature Plants (2022)
-
Genome-wide transcriptome analysis of the orphan crop tef (Eragrostis tef (Zucc.) Trotter) under long-term low calcium stress
Scientific Reports (2022)
-
Composition of Sexual Fluids in Cycas revoluta Ovules During Pollination and Fertilization
The Botanical Review (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.