Abstract
A fundamental feature of immune systems is the ability to distinguish pathogenic from self and commensal elements, and to attack the former but tolerate the latter1. Prokaryotic CRISPR-Cas immune systems defend against phage infection by using Cas nucleases and small RNA guides that specify one or more target sites for cleavage of the viral genome2,3. Temperate phages include viruses that can integrate into the bacterial chromosome, and they can carry genes that provide a fitness advantage to the lysogenic host4,5. However, CRISPR-Cas targeting that relies strictly on DNA sequence recognition provides indiscriminate immunity both to lytic and lysogenic infection by temperate phages6—compromising the genetic stability of these potentially beneficial elements altogether. Here we show that the Staphylococcus epidermidis CRISPR-Cas system can prevent lytic infection but tolerate lysogenization by temperate phages. Conditional tolerance is achieved through transcription-dependent DNA targeting, and ensures that targeting is resumed upon induction of the prophage lytic cycle. Our results provide evidence for the functional divergence of CRISPR-Cas systems and highlight the importance of targeting mechanism diversity. In addition, they extend the concept of ‘tolerance to non-self’ to the prokaryotic branch of adaptive immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Belkaid, Y. & Hand, T. W. Role of the microbiota in immunity and inflammation. Cell 157, 121–141 (2014)
Barrangou, R. CRISPR-Cas systems and RNA-guided interference. Wiley Interdiscip. Rev. RNA 4, 267–278 (2013)
Sorek, R., Lawrence, C. M. & Wiedenheft, B. CRISPR-mediated adaptive immune systems in bacteria and archaea. Annu. Rev. Biochem. 82, 237–266 (2013)
Brüssow, H., Canchaya, C. & Hardt, W. D. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68, 560–602 (2004)
Cumby, N., Davidson, A. R. & Maxwell, K. L. The moron comes of age. Bacteriophage 2, 225–228 (2012)
Edgar, R. & Qimron, U. The Escherichia coli CRISPR system protects from lambda lysogenization, lysogens, and prophage induction. J. Bacteriol. 192, 6291–6294 (2010)
Brouns, S. J. et al. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 (2008)
Carte, J., Wang, R., Li, H., Terns, R. M. & Terns, M. P. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 22, 3489–3496 (2008)
Makarova, K. S. et al. Evolution and classification of the CRISPR-Cas systems. Nature Rev. Microbiol. 9, 467–477 (2011)
Deveau, H. et al. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 190, 1390–1400 (2008)
Semenova, E. et al. Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. Proc. Natl Acad. Sci. USA 108, 10098–10103 (2011)
Marraffini, L. A. & Sontheimer, E. J. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature 463, 568–571 (2010)
Bikard, D., Hatoum-Aslan, A., Mucida, D. & Marraffini, L. A. CRISPR interference can prevent natural transformation and virulence acquisition during in vivo bacterial infection. Cell Host Microbe 12, 177–186 (2012)
Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L. A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nature Biotechnol. 31, 233–239 (2013)
Johnson, A. D. et al. lambda Repressor and cro–components of an efficient molecular switch. Nature 294, 217–223 (1981)
Nozawa, T. et al. CRISPR inhibition of prophage acquisition in Streptococcus pyogenes. PLoS ONE 6, e19543 (2011)
Hatoum-Aslan, A., Samai, P., Maniv, I., Jiang, W. & Marraffini, L. A. A ruler protein in a complex for antiviral defense determines the length of small interfering CRISPR RNAs. J. Biol. Chem. 288, 27888–27897 (2013)
Kreiswirth, B. N. et al. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305, 709–712 (1983)
Bae, T., Baba, T., Hiramatsu, K. & Schneewind, O. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol. Microbiol. 62, 1035–1047 (2006)
Holt, D. C. et al. A very early-branching Staphylococcus aureus lineage lacking the carotenoid pigment staphyloxanthin. Genome Biol. Evol. 3, 881–895 (2011)
Deng, L., Garrett, R. A., Shah, S. A., Peng, X. & She, Q. A novel interference mechanism by a type IIIB CRISPR-Cmr module in Sulfolobus. Mol. Microbiol. 87, 1088–1099 (2013)
Gasiunas, G., Barrangou, R., Horvath, P. & Siksnys, V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl Acad. Sci. USA 109, E2579–E2586 (2012)
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012)
Marraffini, L. A. & Sontheimer, E. J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322, 1843–1845 (2008)
Jiang, W. et al. Dealing with the evolutionary downside of CRISPR immunity: bacteria and beneficial plasmids. PLoS Genet. 9, e1003844 (2013)
Manica, A., Zebec, Z., Steinkellner, J. & Schleper, C. Unexpectedly broad target recognition of the CRISPR-mediated virus defence system in the archaeon Sulfolobus solfataricus. Nucleic Acids Res. 41, 10509–10517 (2013)
Westra, E. R. et al. CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3. Mol. Cell 46, 595–605 (2012)
Nudler, E. RNA polymerase active center: the molecular engine of transcription. Annu. Rev. Biochem. 78, 335–361 (2009)
Hale, C. R. et al. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell 139, 945–956 (2009)
Zhang, J. et al. Structure and Mechanism of the CMR Complex for CRISPR-Mediated Antiviral Immunity. Mol. Cell 45, 303–313 (2012)
Moore, S. D. & Prevelige, P. E., Jr A P22 scaffold protein mutation increases the robustness of head assembly in the presence of excess portal protein. J. Virol. 76, 10245–10255 (2002)
Hatoum-Aslan, A., Maniv, I., Samai, P. & Marraffini, L. A. Genetic Characterization of antiplasmid immunity through a type III-A CRISPR-Cas system. J. Bacteriol. 196, 310–317 (2014)
Helle, L. et al. Vectors for improved Tet repressor-dependent gradual gene induction or silencing in Staphylococcus aureus. Microbiology 157, 3314–3323 (2011)
Horinouchi, S. & Weisblum, B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J. Bacteriol. 150, 804–814 (1982)
Bae, T. & Schneewind, O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55, 58–63 (2006)
Horton, R. M. In vitro recombination and mutagenesis of DNA: SOEing together tailor-made genes. Methods Mol. Biol. 15, 251–261 (1993)
Simpson, J. T. et al. ABySS: a parallel assembler for short read sequence data. Genome Res. 19, 1117–1123 (2009)
Acknowledgements
We acknowledge T. Bae for providing strains; B. Levin, J. Modell and P. Samai for discussion of the paper, and A. Hatoum-Aslan, as well the Torres laboratory (New York University), for their optimization of RNA preparation protocols for S. aureus. We also thank J. Chen of the Novick laboratory for advice on the construction of temperate phages with a selectable marker. Finally, we acknowledge The Rockefeller University Genomics Resource Center core facility for performing the next-generation sequencing in this work. L.A.M. is supported by the Searle Scholars Program, the Rita Allen Scholars Program, an Irma T. Hirschl Award, a Sinsheimer Foundation Award and a National Institutes of Health Director’s New Innovator Award (1DP2AI104556-01).
Author information
Authors and Affiliations
Contributions
G.W.G. and L.A.M. designed experiments. Research was done by G.W.G. W.J. constructed the pWJ40 and pWJ153 plasmids and performed the plasmid-curing experiment. D.B. constructed plasmid pDB184, assisted with phage de novo assembly and provided the reads per million normalization script for RNA sequencing data. G.W.G. and L.A.M. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
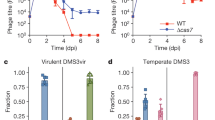
Extended Data Figure 1 Characterization of spacer 32T isolates lysogenized with ΦNM1-ErmR.
a, ΦNM2 sensitivity assay. Eight randomly selected ΦNM1-ErmR lysogen clones were re-streaked through the indicated ΦNM2-seeded region from top to bottom (1–8); C, sensitive ΦNM1-ErmR lysogen harbouring the pGG3 control plasmid. b, PCR amplification of the CRISPR array (upper panel) and spacer 32T target region (lower panel) for the strains tested in a. The pGG3 control lysogen (C) lacks a phage-targeting spacer in its CRISPR array. Size markers of 1 kb and 0.5 kb are indicated. All eight PCR products for the target region were sequenced by the Sanger method and no mutations were found (data not shown). c, Plaque-forming potential of filtered supernatants from spacer 32T lysogen overnight cultures inoculated in triplicate. Plaque-forming units were enumerated on soft agar lawns of RN4220 harbouring either the pGG3 control (C) or spacer 32T CRISPR plasmids. Dotted line represents the limit of detection for this assay. d, ΦNM2 plaquing efficiency on soft agar lawns of an additional six randomly selected ΦNM1-ErmR lysogen clones isolated during infection of RN4220/spacer 32T (9–14); a ΦNM1-ErmR lysogen harbouring the pGG3 control plasmid was also tested (-C/L). Plaquing efficiency on the non-lysogenic indicator strain harbouring pGG3 is shown for comparison (-C). Error bars, mean ± s.d. (n = 3). a, b, Single experiments performed for 8 of 32 isolates.
Extended Data Figure 2 Characterization of spacer 32T isolates lysogenized with ΦNM4.
a, Visualization of TB4-derived strains grown on egg-yolk agar. Integration of ΦNM4 within the geh locus of TB4 results in strongly reduced lipase secretion, enabling a screen for ΦNM4 lysogenization with spacer 32T. Right-most lanes display two lipase-negative isolates from the lysogenization screen; picture is representative of five technical replicates for each isolate. b, ΦNM2 sensitivity assay. Strains shown in a were re-streaked through the indicated ΦNM2-seeded region from top to bottom. The pGG3 lysogen and spacer 32T non-lysogen in the two left-most lanes serve as sensitive and insensitive controls, respectively. Picture is representative of three technical replicates for each isolate. c, ΦNM2 plaquing efficiency on soft agar lawns of the strains analysed in a and b. 32T(L1) and 32T(L2) refer to the two ΦNM4 lysogens isolated during the spacer 32T egg-yolk screen. Error bars, mean ± s.d. (n = 3).
Extended Data Figure 3 Visualization of ΦNM1 transcription profiles 6, 15, 30 and 45 min after infection (MOI 20).
Rightward and leftward expression values are plotted as blue and fuchsia lines, respectively, in reads per million (RPM). Position of relevant spacer targets are indicated with vertical solid lines. The dotted line with arrowheads marks the position of the central promoter. To improve readability, all curves were smoothened by plotting the average reads per million values over a 500 bp sliding-window. To the left of the central promoter, rightward expression is comparable to leftward expression by 30 min after infection, consistent with the strand-independent targeting observed for this region.
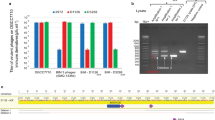
Extended Data Figure 4 Type II CRISPR-Cas targeting in S. aureus prevents both lytic and lysogenic infection.
a, Plaquing efficiency of ΦNM1 and ΦNM1γ6 on lawns of RN4220 harbouring type II-A CRISPR-Cas plasmids as indicated. The parental vector, pDB184, serves as a non-targeting control. b, ΦNM1-ErmR lysogenization of RN4220 harbouring either the spacer 43B-tII, 4B-tII, or non-targeting type II-A CRISPR plasmids. c, ΦNM2 sensitivity assay for seven randomly selected ΦNM1-ErmR lysogen clones isolated during infection of RN4220/spacer 43B-tII (1–7). For comparison, a resistant non-lysogen harbouring the spacer 43B-tII plasmid and a sensitive lysogen harbouring the pDB184 plasmid were included as controls (respectively, C+ and C−). Picture represents a single experiment for 7 of 22 isolates. d, ΦNM2 plaquing efficiency on soft agar lawns for an additional six randomly selected ΦNM1-ErmR lysogen clones isolated during infection of RN4220/spacer 43B-tII (8–13); a ΦNM1-ErmR lysogen harbouring the pDB184 plasmid is also tested (-C/L). For comparison, plaquing efficiency of ΦNM2 on the non-lysogenic indicator strain harbouring pDB184 or the targeting spacer 43B-tII plasmid are also shown (−C and +C, respectively). e, Agarose gel electrophoresis of plasmid DNA purified from isolates 8–13 and the parental spacer 43B-tII strain (C). The symbols + or − indicate the presence or absence of treatment with the BamHI restriction enzyme, which produces two bands for the wild-type spacer 43B-tII plasmid: 5367 bp and 3972 bp. Size markers correspond to 10 kb, 3 kb and 0.5 kb bands of the 1 kb DNA ladder from NEB. f, Colony PCR spanning the type II CRISPR array for isolates 8–13. Spacer 43B-tII plasmid DNA was used as a template for the control (C). Size markers of 3 kb and 0.5 kb are indicated. g, Colony PCR spanning the target region for isolates 8–13 and a ΦNM1-ErmR lysogen harbouring the pDB184 control plasmid (C). Isolates 10 and 11 harbour identical deletions within the prophage that remove the target region (see below). Size markers of 3 kb and 0.5 kb are indicated. The presence of attL and attR prophage integration arms was also verified independently for each isolate using PCR (data not shown). h, Location of the 16,985 bp deletion identified within the prophage harboured by isolates 10 and 11 (shaded grey box). The location and orientation of the ermC insertion cassette is also shown (blue arrow). Deletion was mapped by primer walking. An ∼9.1 kb product spanning the deletion was ultimately amplified using primers oGG6 and oGG241, and the deletion junction was sequenced by the Sanger method using oGG245. A perfect 14 bp direct repeat micro-homology flanks the deletion. i, Plaque-forming potential of overnight culture supernatants from isolates 8, 10 and 11. Supernatants were plated by the soft agar method with RN4220 cells harbouring the non-targeting pDB184 control plasmid as an indicator strain. Supernatants were also plated with spacer 43B-tII targeting lawns, yielding no detectable plaque-forming units. Isolate 8 appears to exhibit wild-type levels of spontaneous prophage induction (compare with pGG3 control in Fig. 4a). No plaque-forming units were detected from the supernatants of isolates 10 and 11 whatsoever, presumably resulting from their deletion of genes essential for prophage induction, including the ORF 43 major capsid protein. Dotted line represents the limit of detection for this assay. Error bars, mean ± s.d. (n = 3). e–g, Single experiments for 6 of 22 isolates.
Extended Data Figure 5 Visualization of transcription profiles for ΦNM1γ6 and the ΦNM1 prophage.
Graphical presentation is the same as in Extended Data Figure 3. a, ΦNM1γ6 transcription profiles 6 and 15 min after infection (MOI 20). Comparison with ΦNM1 samples at equivalent time points (Extended Data Fig. 3) reveals a marked decrease in leftward transcription to the left of the central promoter region. We calculated the fold-change in reads per million between ΦNM1 and ΦNM1γ6 samples 15 min after infection. Leftward expression within the region bounded by the start of the genome and the central promoter was reduced 32-fold, while only a fourfold reduction in leftward expression was observed overall. Meanwhile, rightward expression was reduced fourfold both overall and in this region. This suggests an approximately eightfold net reduction in leftward transcription originating from the central promoter. b, ΦNM1 prophage transcription profiles. Strong leftward transcription originates from the central promoter and a few upstream regions, which are presumed to be important for lysogenic maintenance. Rightward transcription was weaker than leftward transcription as expected, but not absent. Given the strength of rightward transcription observed during the lytic cycle (Extended Data Fig. 3), however, this transcription may originate from a subpopulation of cells undergoing prophage induction, rather than the stable lysogen majority.
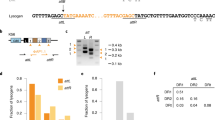
Extended Data Figure 6 Detection of transcription across target insertions for the pNes(wt-d) and pNes(wt-i) plasmids.
For each target plasmid24, reverse transcription was performed in both directions with DNase-treated total RNA from RN4220 cells harbouring the indicated plasmids, using either forward or reverse primers for cDNA synthesis in two separate reactions. PCR was performed on cDNA products, or plasmid DNA templates for control (C) lanes. The symbols + or − indicate the presence or absence of reverse transcriptase enzyme in the reverse transcription reaction mixture used for PCR. Size markers of 500 bp and 100 bp are indicated. Picture is representative of a single technical replicate.
Extended Data Figure 7 Reverse CRISPR-immunity assays using inverted chromosomal target insertions or type II CRISPR-Cas plasmids.
Values represent the average transformation efficiency of three transformations in colony-forming units per microgram of plasmid DNA transformed. ATc, anhydrotetracycline at 0.5 µg ml−1. Dotted lines represent the limit of detection for these assays. a, Reverse CRISPR-immunity assays using inverted target vector insertions and spacer 43T or 43B plasmid DNA. Inversion of the attP motif (‘Inv-attP-’) for forward and reverse insertion vectors causes integration in the opposite orientation relative to the chromosomal origin of replication. b, Reverse CRISPR-immunity assays using type II-A CRISPR plasmid DNA to transform strains from Fig. 3b. The pDB184 parent vector serves as a non-targeting control. Error bars, mean ± s.d. (n = 3).
Extended Data Figure 8 Infection with ΦNM1 in liquid culture.
Growth curves of RN4220 cells harbouring the indicated CRISPR plasmids were infected at time zero with ΦNM1 at an MOI of 10 (a) or 100 (b). Growth of uninfected RN4220/pGG3 cultures is also shown (dotted red lines).
Extended Data Figure 9 Immunity to ΦNM1γ6 in liquid culture is unaffected by the presence of a tolerated chromosomal target.
Growth curves of the indicated chromosomal insertion strains from Fig. 3 harbouring either spacer 43T or pGG3 CRISPR plasmids, in the absence (dotted lines) or presence (solid lines) of ΦNM1γ6 addition at an MOI of 10. Black arrow denotes the time of phage addition; no ATc induction is used in this assay. The presence of a chromosomal target for spacer 43T has no discernable effect on culture growth during spacer 43T-mediated immunity to ΦNM1γ6 (compare solid green and blue lines).
Extended Data Figure 10 Inducible curing of a target plasmid.
a, Diagram of plasmids used in the plasmid-curing experiment. The pGG3 CRISPR plasmid harbours a single spacer (‘spc1’) targeting a sequence (‘prtspc1’) inserted downstream of the Pxyl/tet*-inducible promoter in pWJ153. b, Agarose gel electrophoresis of linearized plasmid DNA purified both from anhydrotetracycline-treated (+ATc) and untreated (−ATc) cultures at the indicated time points. Size markers of 10 kb, 5 kb and 4 kb are indicated. Picture is representative of a single technical replicate. c, Colony-forming units recovered from cultures analysed in b at each time point. Cells were plated with selection for either CmR colony-forming units (green) or CmR, ErmR colony-forming units (blue). Targeting of the pWJ153 plasmid via induction with ATc (filled circles) is accompanied by a severe drop in erythromycin-resistant colony-forming units relative to untreated cultures (open circles). Error bars, mean ± s.d. (n = 3).
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-3 and Supplementary Sequence 1. (PDF 1441 kb)
Rights and permissions
About this article
Cite this article
Goldberg, G., Jiang, W., Bikard, D. et al. Conditional tolerance of temperate phages via transcription-dependent CRISPR-Cas targeting. Nature 514, 633–637 (2014). https://doi.org/10.1038/nature13637
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13637
This article is cited by
-
Arbitrium communication controls phage lysogeny through non-lethal modulation of a host toxin–antitoxin defence system
Nature Microbiology (2024)
-
Bacterial cGAS senses a viral RNA to initiate immunity
Nature (2023)
-
RNA-targeting CRISPR–Cas systems
Nature Reviews Microbiology (2023)
-
Population genomics of Lacticaseibacillus paracasei: pan-genome, integrated prophage, antibiotic resistance, and carbohydrate utilization
World Journal of Microbiology and Biotechnology (2023)
-
Structural rearrangements allow nucleic acid discrimination by type I-D Cascade
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.