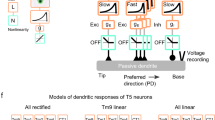
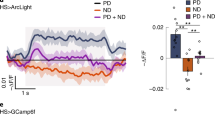
Abstract
The extraction of directional motion information from changing retinal images is one of the earliest and most important processing steps in any visual system. In the fly optic lobe, two parallel processing streams have been anatomically described, leading from two first-order interneurons, L1 and L2, via T4 and T5 cells onto large, wide-field motion-sensitive interneurons of the lobula plate1. Therefore, T4 and T5 cells are thought to have a pivotal role in motion processing; however, owing to their small size, it is difficult to obtain electrical recordings of T4 and T5 cells, leaving their visual response properties largely unknown. We circumvent this problem by means of optical recording from these cells in Drosophila, using the genetically encoded calcium indicator GCaMP5 (ref. 2). Here we find that specific subpopulations of T4 and T5 cells are directionally tuned to one of the four cardinal directions; that is, front-to-back, back-to-front, upwards and downwards. Depending on their preferred direction, T4 and T5 cells terminate in specific sublayers of the lobula plate. T4 and T5 functionally segregate with respect to contrast polarity: whereas T4 cells selectively respond to moving brightness increments (ON edges), T5 cells only respond to moving brightness decrements (OFF edges). When the output from T4 or T5 cells is blocked, the responses of postsynaptic lobula plate neurons to moving ON (T4 block) or OFF edges (T5 block) are selectively compromised. The same effects are seen in turning responses of tethered walking flies. Thus, starting with L1 and L2, the visual input is split into separate ON and OFF pathways, and motion along all four cardinal directions is computed separately within each pathway. The output of these eight different motion detectors is then sorted such that ON (T4) and OFF (T5) motion detectors with the same directional tuning converge in the same layer of the lobula plate, jointly providing the input to downstream circuits and motion-driven behaviours.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bausenwein, B., Dittrich, A. P. M. & Fischbach, K. F. The optic lobe of Drosophila melanogaster II. Sorting of retinotopic pathways in the medulla. Cell Tissue Res. 267, 17–28 (1992)
Akerboom, J. et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819–13840 (2012)
Cajal, S. R. & Sanchez, D. Contribucion al conocimiento de los centros nerviosos de los insectos (Imprenta de Hijos de Nicholas Moja, 1915)
Strausfeld, N. J. Atlas of an Insect Brain (Springer, 1976)
Fischbach, K. F. & Dittrich, A. P. M. The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res. 258, 441–475 (1989)
Reichardt, W. Autocorrelation, a principle for the evaluation of sensory information by the central nervous system. In Sensory Communication (ed. Rosenblith, W. A. ) 303–317 (MIT Press and John Wiley & Sons, 1961)
Borst, A., Haag, J. & Reiff, D. F. Fly motion vision. Annu. Rev. Neurosci. 33, 49–70 (2010)
Buchner, E., Buchner. S & Buelthoff, I. Deoxyglucose mapping of nervous activity induced in Drosophila brain by visual movement. 1. Wildtype. J. Comp. Physiol. 155, 471–483 (1984)
Strausfeld, N. J. & Lee, J. K. Neuronal basis for parallel visual processing in the fly. Vis. Neurosci. 7, 13–33 (1991)
Schnell, B., Raghu, V. S., Nern, A. & Borst, A. Columnar cells necessary for motion responses of wide-field visual interneurons in Drosophila. J. Comp. Physiol. A 198, 389–395 (2012)
Douglass, J. K. & Strausfeld, N. J. Visual motion-detection circuits in flies: Parallel direction- and non-direction-sensitive pathways between the medulla and lobula plate. J. Neurosci. 16, 4551–4562 (1996)
Franceschini, N., Riehle, A. & Le Nestour, A. Directionally selective motion detection by insect neurons. In Facets of Vision (ed. Stavenga, H. ) 360–390 (Springer, 1989)
Joesch, M., Schnell, B., Raghu, S. V., Reiff, D. F. & Borst, A. ON and OFF pathways in Drosophila motion vision. Nature 468, 300–304 (2010)
Pfeiffer, B. D. et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl Acad. Sci. USA 105, 9715–9720 (2008)
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990)
Oyster, C. W. & Barlow, H. B. Direction-selective units in rabbit retina: distribution of preferred directions. Science 155, 841–842 (1967)
Sweeney, S. T., Broadie, K., Keane, J., Niemann, H. & O’Kane, C. J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351 (1995)
Seelig, J. D. et al. Two-photon calcium imaging from head-fixed Drosophila during optomotor walking behavior. Nature Methods 7, 535–540 (2010)
Clark, D. A., Bursztyn, L., Horowitz, M. A., Schnitzer, M. J. & Clandinin, T. R. Defining the computational structure of the motion detector in Drosophila. Neuron 70, 1165–1177 (2011)
Egelhaaf, M. & Borst, A. Calcium accumulation in visual interneurons of the fly: Stimulus dependence and relationship to membrane potential. J. Neurophysiol. 73, 2540–2552 (1995)
Joesch, M., Plett, J., Borst, A. & Reiff, D. F. Response properties of motion-sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Curr. Biol. 18, 368–374 (2008)
Schnell, B. et al. Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J. Neurophysiol. 103, 1646–1657 (2010)
Borst, A. & Egelhaaf, M. Direction selectivity of fly motion-sensitive neurons is computed in a two-stage process. Proc. Natl Acad. Sci. USA 87, 9363–9367 (1990)
Single, S., Haag, J. & Borst, A. Dendritic computation of direction selectivity and gain control in visual interneurons. J. Neurosci. 17, 6023–6030 (1997)
Eichner, H., Joesch, M., Schnell, B., Reiff, D. F. & Borst, A. Internal structure of the fly elementary motion detector. Neuron 70, 1155–1164 (2011)
Joesch, M., Weber, F., Eichner, H. & Borst, A. Functional specialization of parallel motion detection circuits in the fly. J. Neurosci. 33, 902–905 (2013)
Egelhaaf, M. & Borst, A. Are there separate ON and OFF channels in fly motion vision? Vis. Neurosci. 8, 151–164 (1992)
Takemura, S. Y., Lu, Z. & Meinertzhagen, I. A. Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla. J. Comp. Neurol. 509, 493–513 (2008)
Euler, T., Detwiler, P. B. & Denk, W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418, 845–852 (2002)
Pologruto, T. A., Sabatini, B. L. & Svoboda, K. ScanImage: Flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003)
Jenett, A. et al. A Gal4-driver line resource for Drosophila neurobiology. Cell Rep. 2, 991–1001 (2012)
Reiff, D. F., Plett, J., Mank, M., Griesbeck, O. & Borst, A. Visualizing retinotopic half-wave rectified input to the motion detection circuitry of Drosophila. Nature Neurosci. 13, 973–978 (2010)
Euler, T. et al. Eyecup scope—optical recording of light stimulus-evoked fluorescence signals in the retina. Pfluger Arch. 457, 1393–1414 (2009)
Reiser, M. B. & Dickinson, M. H. A modular display system for insect behavioral neuroscience. J. Neurosci. Methods 167, 127–139 (2008)
Acknowledgements
We thank L. Looger, J. Simpson, V. Jayaraman and the Janelia GECI team for making and providing us with the GCaMP5 flies before publication; J. Plett for designing and engineering the LED arena; C. Theile, W. Essbauer and M. Sauter for fly work; and A. Mauss, F. Gabbiani and T. Bonhoeffer for critically reading the manuscript. This work was in part supported by the Deutsche Forschungsgemeinschaft (SFB 870). M.S.M., G.A., E.S., M.M., A.L., A.Ba and A.Bo are members of the Graduate School of Systemic Neurosciences.
Author information
Authors and Affiliations
Contributions
M.S.M. and J.H. jointly performed and, together with A.Bo., evaluated all calcium imaging experiments. G.A., E.S. and M.M. recorded from tangential cells. A.L., T.S. and A.Ba. performed the behavioural experiments. G.R., B.D. and A.N. generated the driver lines and characterized their expression pattern. D.F.R. performed preliminary imaging experiments. E.H. helped with programming and developed the PMT shielding for the two-photon microscope. A.Bo. designed the study and wrote the manuscript with the help of all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-2 and additional references. (PDF 485 kb)
Rights and permissions
About this article
Cite this article
Maisak, M., Haag, J., Ammer, G. et al. A directional tuning map of Drosophila elementary motion detectors. Nature 500, 212–216 (2013). https://doi.org/10.1038/nature12320
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12320
This article is cited by
-
Localized calcium transients in phragmoplast regulate cytokinesis of tobacco BY-2 cells
Plant Cell Reports (2024)
-
Mechanismen und Herausforderungen des Bewegungssehens
BIOspektrum (2023)
-
Direction Selectivity of TmY Neurites in Drosophila
Neuroscience Bulletin (2023)
-
Neural Circuit Mechanisms Involved in Animals’ Detection of and Response to Visual Threats
Neuroscience Bulletin (2023)
-
A biophysical account of multiplication by a single neuron
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.