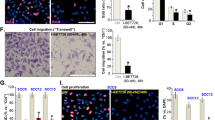
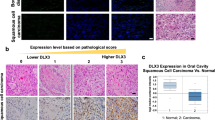
Abstract
Calcineurin inhibitors such as cyclosporin A (CsA) are the mainstay of immunosuppressive treatment for organ transplant recipients. Squamous cell carcinoma (SCC) of the skin is a major complication of treatment with these drugs, with a 65 to 100-fold higher risk than in the normal population1. By contrast, the incidence of basal cell carcinoma (BCC), the other major keratinocyte-derived tumour of the skin, of melanoma and of internal malignancies increases to a significantly lesser extent1. Here we report that genetic and pharmacological suppression of calcineurin/nuclear factor of activated T cells (NFAT) function promotes tumour formation in mouse skin and in xenografts, in immune compromised mice, of H-rasV12 (also known as Hras1)-expressing primary human keratinocytes or keratinocyte-derived SCC cells. Calcineurin/NFAT inhibition counteracts p53 (also known as TRP53)-dependent cancer cell senescence, thereby increasing tumorigenic potential. ATF3, a member of the ‘enlarged’ AP-1 family, is selectively induced by calcineurin/NFAT inhibition, both under experimental conditions and in clinically occurring tumours, and increased ATF3 expression accounts for suppression of p53-dependent senescence and enhanced tumorigenic potential. Thus, intact calcineurin/NFAT signalling is critically required for p53 and senescence-associated mechanisms that protect against skin squamous cancer development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Euvrard, S., Kanitakis, J. & Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 348, 1681–1691 (2003)
Horsley, V., Aliprantis, A. O., Polak, L., Glimcher, L. H. & Fuchs, E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132, 299–310 (2008)
Mammucari, C. et al. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev. Cell 8, 665–676 (2005)
Santini, M. P., Talora, C., Seki, T., Bolgan, L. & Dotto, G. P. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21WAF1/CIP1 expression in keratinocyte differentiation. Proc. Natl Acad. Sci. USA 98, 9575–9580 (2001)
Aramburu, J. et al. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285, 2129–2133 (1999)
Boukamp, P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis 26, 1657–1667 (2005)
Rheinwald, J. G. & Beckett, M. A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res. 41, 1657–1663 (1981)
Brash, D. E. et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl Acad. Sci. USA 88, 10124–10128 (1991)
Burns, J. E. et al. Gene mutations and increased levels of p53 protein in human squamous cell carcinomas and their cell lines. Br. J. Cancer 67, 1274–1284 (1993)
Kolev, V. et al. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nature Cell Biol. 10, 902–911 (2008)
Adorno, M. et al. A mutant-p53/Smad complex opposes p63 to empower TGFβ-induced metastasis. Cell 137, 87–98 (2009)
Gaiddon, C., Lokshin, M., Ahn, J., Zhang, T. & Prives, C. A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol. Cell. Biol. 21, 1874–1887 (2001)
Keyes, W. M. et al. p63 deficiency activates a program of cellular senescence and leads to accelerated aging. Genes Dev. 19, 1986–1999 (2005)
Nguyen, B. C. et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 20, 1028–1042 (2006)
Finkel, T., Serrano, M. & Blasco, M. A. The common biology of cancer and ageing. Nature 448, 767–774 (2007)
Krizhanovsky, V. et al. Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology. Cold Spring Harb. Symp. Quant. Biol. 73, 513–522 (2008)
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo . Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995)
Wang, A. et al. Epidermal hyperplasia and oral carcinoma in mice overexpressing the transcription factor ATF3 in basal epithelial cells. Mol. Carcinog. 46, 476–487 (2007)
Neal, J. W. & Clipstone, N. A. A constitutively active NFATc1 mutant induces a transformed phenotype in 3T3-L1 fibroblasts. J. Biol. Chem. 278, 17246–17254 (2003)
Cano, E., Canellada, A., Minami, T., Iglesias, T. & Redondo, J. M. Depolarization of neural cells induces transcription of the Down syndrome critical region 1 isoform 4 via a calcineurin/nuclear factor of activated T cells-dependent pathway. J. Biol. Chem. 280, 29435–29443 (2005)
Li, A. & Kaur, P. FACS enrichment of human keratinocyte stem cells. Methods Mol. Biol. 289, 87–96 (2005)
Quintana, E. et al. Efficient tumour formation by single human melanoma cells. Nature 456, 593–598 (2008)
Locke, M., Heywood, M., Fawell, S. & Mackenzie, I. C. Retention of intrinsic stem cell hierarchies in carcinoma-derived cell lines. Cancer Res. 65, 8944–8950 (2005)
Prince, M. E. & Ailles, L. E. Cancer stem cells in head and neck squamous cell cancer. J. Clin. Oncol. 26, 2871–2875 (2008)
Mancini, M. & Toker, A. NFAT proteins: emerging roles in cancer progression. Nature Rev. Cancer 9, 810–820 (2009)
Baek, K. H. et al. Down’s syndrome suppression of tumour growth and the role of the calcineurin inhibitor DSCR1. Nature 459, 1126–1130 (2009)
Ryeom, S. et al. Targeted deletion of the calcineurin inhibitor DSCR1 suppresses tumor growth. Cancer Cell 13, 420–431 (2008)
Roderick, H. L. & Cook, S. J. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nature Rev. Cancer 8, 361–375 (2008)
Lefort, K. et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKα kinases. Genes Dev. 21, 562–577 (2007)
Zheng, Y. et al. Organogenesis from dissociated cells: generation of mature cycling hair follicles from skin-derived cells. J. Invest. Dermatol. 124, 867–876 (2005)
Lazarov, M. et al. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nature Med. 8, 1105–1114 (2002)
Tamura, K. et al. Stress response gene ATF3 is a target of c-myc in serum-induced cell proliferation. EMBO J. 24, 2590–2601 (2005)
Nguyen, B. C. et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 20, 1028–1042 (2006)
Malanchi, I. et al. Cutaneous cancer stem cell maintenance is dependent on β-catenin signalling. Nature 452, 650–653 (2008)
Chen, C. R., Kang, Y. & Massague, J. Defective repression of c-myc in breast cancer cells: a loss at the core of the transforming growth factor beta growth arrest program. Proc. Natl Acad. Sci. USA 98, 992–999 (2001)
Topley, G. I., Okuyama, R., Gonzales, J. G., Conti, C. & Dotto, G. P. p21WAF1/Cip1 functions as a suppressor of malignant skin tumor formation and a determinant of keratinocyte stem-cell potential. Proc. Natl Acad. Sci. USA 96, 9089–9094 (1999)
Dotto, G. P., Moellmann, G., Ghosh, S., Edwards, M. & Halaban, R. Transformation of murine melanocytes by basic fibroblast growth factor cDNA and oncogenes and selective suppression of the transformed phenotype in a reconstituted cutaneous environment. J. Cell Biol. 109, 3115–3128 (1989)
Dotto, G. P., Weinberg, R. A. & Ariza, A. Malignant transformation of mouse primary keratinocytes by Harvey sarcoma virus and its modulation by surrounding normal cells. Proc. Natl Acad. Sci. USA 85, 6389–6393 (1988)
Acknowledgements
We thank P. Khavari, S. Kitajima, N. Clipstone and G. Crabtree for gift of retroviruses, W. Austen for human skin material, C. Brisken and C. Missero for careful reading of the manuscript, and E. Castillo for sequencing of the ras and p53 genes. This work was supported by grants from NIH (AR054856 and AR39190), the Swiss National Foundation (311003A-122281/1), Oncosuisse (OCS-02361-02-2009), the European Union (Epistem, Sixth Framework Program, LSHB-CT-2005-019067) and, in part, by a grant to S.C. by the Korean Government Foundation (KRF-2007-013-E00044) and to G.F.L.H. by the Olga-Mayenfisch-Stiftung.
Author information
Authors and Affiliations
Contributions
B-C.N., P.D, S.C, Y.B., K.L. and G.F.L.H. performed research and analysed data; X.W. and G.P.D. designed and performed research, analysed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-14 with legends, Supplementary Tables I-III and References. (PDF 41972 kb)
Rights and permissions
About this article
Cite this article
Wu, X., Nguyen, BC., Dziunycz, P. et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature 465, 368–372 (2010). https://doi.org/10.1038/nature08996
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08996
This article is cited by
-
Advances in cutaneous squamous cell carcinoma
Nature Reviews Cancer (2023)
-
mTORC1 induces plasma membrane depolarization and promotes preosteoblast senescence by regulating the sodium channel Scn1a
Bone Research (2022)
-
A carbazole compound, 9-ethyl-9H-carbazole-3-carbaldehyde, plays an antitumor function through reactivation of the p53 pathway in human melanoma cells
Cell Death & Disease (2021)
-
ATF-3 expression inhibits melanoma growth by downregulating ERK and AKT pathways
Laboratory Investigation (2021)
-
Loss of activating transcription factor 3 prevents KRAS-mediated pancreatic cancer
Oncogene (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.