Abstract
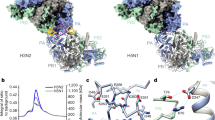
The recent emergence of highly pathogenic avian (H5N1) influenza viruses, their epizootic and panzootic nature, and their association with lethal human infections have raised significant global health concerns1,2. Several studies have underlined the importance of non-structural protein NS1 in the increased pathogenicity and virulence of these strains3,4. NS1, which consists of two domains—a double-stranded RNA (dsRNA) binding domain5,6 and the effector domain7, separated through a linker—is an antagonist of antiviral type-I interferon response in the host8,9. Here we report the X-ray structure of the full-length NS1 from an H5N1 strain (A/Vietnam/1203/2004) that was associated with 60% of human deaths in an outbreak in Vietnam1,2. Compared to the individually determined structures of the RNA binding domain and the effector domain from non-H5N1 strains, the RNA binding domain within H5N1 NS1 exhibits modest structural changes, while the H5N1 effector domain shows significant alteration, particularly in the dimeric interface. Although both domains in the full-length NS1 individually participate in dimeric interactions, an unexpected finding is that these interactions result in the formation of a chain of NS1 molecules instead of distinct dimeric units. Three such chains in the crystal interact with one another extensively to form a tubular organization of similar dimensions to that observed in the cryo-electron microscopy images of NS1 in the presence of dsRNA. The tubular oligomeric organization of NS1, in which residues implicated in dsRNA binding face a 20-Å-wide central tunnel, provides a plausible mechanism for how NS1 sequesters varying lengths of dsRNA, to counter cellular antiviral dsRNA response pathways, while simultaneously interacting with other cellular ligands during an infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Abdel-Ghafar, A. N. et al. Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358, 261–273 (2008)
Beigel, J. H. et al. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353, 1374–1385 (2005)
Seo, S. H., Hoffmann, E. & Webster, R. G. The NS1 gene of H5N1 influenza viruses circumvents the host anti-viral cytokine responses. Virus Res. 103, 107–113 (2004)
Seo, S. H., Hoffmann, E. & Webster, R. G. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nature Med. 8, 950–954 (2002)
Chien, C. Y. et al. A novel RNA-binding motif in influenza A virus non-structural protein 1. Nature Struct. Biol. 4, 891–895 (1997)
Liu, J. et al. Crystal structure of the unique RNA-binding domain of the influenza virus NS1 protein. Nature Struct. Biol. 4, 896–899 (1997)
Bornholdt, Z. A. & Prasad, B. V. X-ray structure of influenza virus NS1 effector domain. Nature Struct. Mol. Biol. 13, 559–560 (2006)
Garcia-Sastre, A. et al. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252, 324–330 (1998)
Wang, X. et al. Influenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferon. J. Virol. 74, 11566–11573 (2000)
Kochs, G., Garcia-Sastre, A. & Martinez-Sobrido, L. Multiple anti-interferon actions of the influenza A virus NS1 protein. J. Virol. 81, 7011–7021 (2007)
Min, J. Y. & Krug, R. M. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: Inhibiting the 2'-5′ oligo (A) synthetase/RNase L pathway. Proc. Natl Acad. Sci. USA 103, 7100–7105 (2006)
Lu, Y., Wambach, M., Katze, M. G. & Krug, R. M. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology 214, 222–228 (1995)
Hale, B. G. et al. Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling. Proc. Natl Acad. Sci. USA 103, 14194–14199 (2006)
Nemeroff, M. E. et al. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′end formation of cellular pre-mRNAs. Mol. Cell 1, 991–1000 (1998)
Twu, K. Y. et al. The CPSF30 binding site on the NS1A protein of influenza A virus is a potential antiviral target. J. Virol. 80, 3957–3965 (2006)
Wang, W. et al. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA 5, 195–205 (1999)
Hale, B. G., Barclay, W. S., Randall, R. E. & Russell, R. J. Structure of an avian influenza A virus NS1 protein effector domain. Virology 378, 1–5 (2008)
Li, K. S. et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430, 209–213 (2004)
Noah, D. L., Twu, K. Y. & Krug, R. M. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3′ end processing of cellular pre-mRNAS. Virology 307, 386–395 (2003)
Diebold, S. S. et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424, 324–328 (2003)
Wang, Q. & Carmichael, G. G. Effects of length and location on the cellular response to double-stranded RNA. Microbiol. Mol. Biol. Rev. 68, 432–452 (2004)
Li, W. X. et al. Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. Proc. Natl Acad. Sci. USA 101, 1350–1355 (2004)
Melen, K. et al. Nuclear and nucleolar targeting of influenza A virus NS1 protein: Striking differences between different virus subtypes. J. Virol. 81, 5995–6006 (2007)
Pflugrath, J. W. The finer things in X-ray diffraction data collection. Acta Crystallogr. D 55, 1718–1725 (1999)
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D 62, 72–82 (2006)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Murshudov, G. N., Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 53, 240–255 (1997)
The. CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998)
Emsley, P. & Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Brunger, A. T. Free R value: A novel statistical quantity for assessing the accuracy of crystal structures. Nature 355, 472–475 (1992)
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993)
Dubochet, J. et al. Cryo-electron microscopy of vitrified specimens. Q. Rev. Biophys. 21, 129–228 (1988)
Acknowledgements
We thank P. Palese for providing us clones of A/Vietnam/1203/2004 H5N1 and A/PR8/34 (H1N1) NS1. This work was supported by the NIH (AI36040) and the Robert Welch Foundation (to B.V.V.P.). Z.A.B. acknowledges support from an NIH virology training grant (AI07471). We thank P. Palese, A. Rice, M. Schmid and B. Carrillo for discussions and comments on the manuscript and H. Chen for technical assistance with cryo-EM. We acknowledge the use of cryo-EM facilities at the National Center for Macromolecular Imaging (Baylor College of Medicine) supported by the National Institutes of Health (RR002250 to W. Chiu); we also acknowledge the Center for Advanced Microstructures & Devices (CAMD), Baton Rouge, Los Angeles, and H. Bellamy, and the SBC-CAT 19ID beam line at the Advanced Photon Source (supported by the US Department of Energy, Basic Energy Sciences, Office of Science, under contract no.W-31-109-Eng-38) and its staff for their help during data collection.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1-S5, Supplementary Table S1 and Supplementary References (PDF 610 kb)
Rights and permissions
About this article
Cite this article
Bornholdt, Z., Prasad, B. X-ray structure of NS1 from a highly pathogenic H5N1 influenza virus. Nature 456, 985–988 (2008). https://doi.org/10.1038/nature07444
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07444
This article is cited by
-
Roles of NOLC1 in cancers and viral infection
Journal of Cancer Research and Clinical Oncology (2023)
-
Structural basis for influenza virus NS1 protein block of mRNA nuclear export
Nature Microbiology (2019)
-
Molecular mechanism of influenza A NS1-mediated TRIM25 recognition and inhibition
Nature Communications (2018)
-
Influenza virus infection causes global RNAPII termination defects
Nature Structural & Molecular Biology (2018)
-
Identification of NS1 domains of avian H5N1 influenza virus which influence the interaction with the NOLC1 protein
Virus Genes (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.