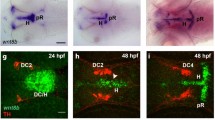
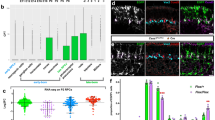
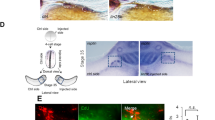
Abstract
How a cell chooses to proliferate or to differentiate is an important issue in stem cell and cancer biology. Drosophila neuroblasts undergo self-renewal with every cell division, producing another neuroblast and a differentiating daughter cell, but the mechanisms controlling the self-renewal/differentiation decision are poorly understood. Here we tested whether cell polarity genes, known to regulate embryonic neuroblast asymmetric cell division1, also regulate neuroblast self-renewal. Clonal analysis in larval brains showed that pins mutant neuroblasts rapidly fail to self-renew, whereas lethal giant larvae (lgl) mutant neuroblasts generate multiple neuroblasts. Notably, lgl pins double mutant neuroblasts all divide symmetrically to self-renew, filling the brain with neuroblasts at the expense of neurons. The lgl pins neuroblasts show ectopic cortical localization of atypical protein kinase C (aPKC), and a decrease in aPKC expression reduces neuroblast numbers, suggesting that aPKC promotes neuroblast self-renewal. In support of this hypothesis, neuroblast-specific overexpression of membrane-targeted aPKC, but not a kinase-dead version, induces ectopic neuroblast self-renewal. We conclude that cortical aPKC kinase activity is a potent inducer of neuroblast self-renewal.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Betschinger, J. & Knoblich, J. A. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14, R674–R685 (2004)
Ohlstein, B., Kai, T., Decotto, E. & Spradling, A. The stem cell niche: theme and variations. Curr. Opin. Cell Biol. 16, 693–699 (2004)
Urbach, R. & Technau, G. M. Neuroblast formation and patterning during early brain development in Drosophila. BioEssays 26, 739–751 (2004)
Datta, S. Control of proliferation activation in quiescent neuroblasts of the Drosophila central nervous system. Development 121, 1173–1182 (1995)
Gateff, E. & Schneiderman, H. A. Developmental capacities of benign and malignant neoplasms of Drosophila. Roux Arch. Dev. Biol. 176, 23–65 (1974)
Ohshiro, T., Yagami, T., Zhang, C. & Matsuzaki, F. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature 408, 593–596 (2000)
Peng, C. Y., Manning, L., Albertson, R. & Doe, C. Q. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature 408, 596–600 (2000)
Albertson, R. & Doe, C. Q. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nature Cell Biol. 5, 166–170 (2003)
Strand, D. et al. The Drosophila lethal(2)giant larvae tumour suppressor protein forms homo-oligomers and is associated with nonmuscle myosin II heavy chain. J. Cell Biol. 127, 1361–1373 (1994)
Betschinger, J., Mechtler, K. & Knoblich, J. A. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422, 326–330 (2003)
Buenzow, D. E. & Holmgren, R. Expression of the Drosophila gooseberry locus defines a subset of neuroblast lineages in the central nervous system. Dev. Biol. 170, 338–349 (1995)
Freeman, M. R. & Doe, C. Q. Asymmetric Prospero localization is required to generate mixed neuronal/glial lineages in the Drosophila CNS. Development 128, 4103–4112 (2001)
Spana, E. P. & Doe, C. Q. The Prospero transcription factor is asymmetrically localized to the cell cortex during neuroblast mitosis in Drosophila. Development 121, 3187–3195 (1995)
Bilder, D., Li, M. & Perrimon, N. Cooperative regulation of cell polarity and growth by Drosophila tumour suppressors. Science 289, 113–116 (2000)
Hutterer, A., Betschinger, J., Petronczki, M. & Knoblich, J. A. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev. Cell 6, 845–854 (2004)
Yamanaka, T. et al. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr. Biol. 13, 734–743 (2003)
Chalmers, A. D. et al. aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development 132, 977–986 (2005)
Rolls, M. M., Albertson, R., Shih, H. P., Lee, C. Y. & Doe, C. Q. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J. Cell Biol. 163, 1089–1098 (2003)
Sotillos, S., Diaz-Meco, M. T., Caminero, E., Moscat, J. & Campuzano, S. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J. Cell Biol. 166, 549–557 (2004)
Caussinus, E. & Gonzalez, C. Induction of tumour growth by altered stem-cell asymmetric division in Drosophila melanogaster. Nature Genet. 37, 1125–1129 (2005)
Klezovitch, O., Fernandez, T. E., Tapscott, S. J. & Vasioukhin, V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 18, 559–571 (2004)
Sanada, K. & Tsai, L. H. G Protein βγ subunits and AGS3 control spindle orientation and asymmetric cell fate of cerebral cortical progenitors. Cell 122, 119–131 (2005)
Yu, F., Morin, X., Cai, Y., Yang, X. & Chia, W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell 100, 399–409 (2000)
Albertson, R., Chabu, C., Sheehan, A. & Doe, C. Q. Scribble protein domain mapping reveals a multistep localization mechanism and domains necessary for establishing cortical polarity. J. Cell Sci. 117, 6061–6070 (2004)
Pearson, B. J. & Doe, C. Q. Regulation of neuroblast competence in Drosophila. Nature 425, 624–628 (2003)
Freeman, M. R., Delrow, J., Kim, J., Johnson, E. & Doe, C. Q. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron 38, 567–580 (2003)
Grosskortenhaus, R., Pearson, B. J., Marusich, A. & Doe, C. Q. Regulation of temporal identity transitions in Drosophila neuroblasts. Dev. Cell 8, 193–202 (2005)
Acknowledgements
We thank J. Knoblich, S. Campuzano, B. Chia, J. Skeath and B. Holmgren for fly stocks and/or antibody reagents; B. Bowerman, J. Eisen, K. Siller and S. Siegrist for comments on the manuscript; and C. Chabu for discussion. C.-Y.L. is supported by a Damon Runynon postdoctoral fellowship. C.Q.D. is supported by the Howard Hughes Medical Institute, where he is an Investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
lgl and pins regulate larval neuroblast numbers. (PDF 132 kb)
Supplementary Figure 2
Apoptosis is not increased in pins mutant brains. (PDF 59 kb)
Supplementary Figure 3
A comparison of neuroblast and GMC markers reveals that lgl pins mutant brains have supernumerary neuroblasts. (PDF 326 kb)
Supplementary Figure 4
aPKC is required for neuroblast self-renewal in lgl mutants. (PDF 120 kb)
Supplementary Data
Gene/protein list (DOC 19 kb)
Supplementary Methods
Additional description of the methods used in this study. (DOC 23 kb)
Supplementary Movie1
Thousands of differentiating GMCs and neurons rapidly down-regulate neuroblast markers and express nuclear Prospero and/or Elav. (MOV 2660 kb)
Supplementary Movie 2
There was a clear increase in neuroblast number in lgl and dlg mutants; and also supernumerary neuroblasts at all stages examined. All extra neuroblasts expressed Deadpan and Miranda neuroblast markers and were proliferative based on their ability to incorporate BrdU. (MOV 2750 kb)
Supplementary Movie3
Gαi zygotic mutants had a complex phenotype, but pins zygotic mutants showed a striking decrease in neuroblast number. (MOV 2450 kb)
Supplementary Movie 4
A novel phenotype was detected, in which the larval brain was full of cells expressing the neuroblast markers Worniu, Miranda and Deadpan, and lacking expression of the neuronal marker Elav. (MOV 2209 kb)
Supplementary Movie 5
Neuroblast-specific expression of aPKC targeted to the plasma membrane with a CAAX prenylation motif (UAS-aPKCCAAXWT) resulted in ectopic cortical aPKC localization, loss of cortical Miranda and a massive increase in the number of neuroblasts. (MOV 2686 kb)
Supplementary Movie 6
Neuroblast-specific expression of aPKC targeted to the plasma membrane with a CAAX prenylation motif (UAS-aPKCCAAXWT) resulted in ectopic cortical aPKC localization, loss of cortical Miranda and a massive increase in the number of neuroblasts. These effects were not observed following overexpression of wild type aPKC or a membrane-targeted kinase-dead aPKC (UAS-aPKCCAAXKD). (MOV 2431 kb)
Supplementary Figure Legends
Text to accompany the above Supplementary Figures. (DOC 21 kb)
Rights and permissions
About this article
Cite this article
Lee, CY., Robinson, K. & Doe, C. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature 439, 594–598 (2006). https://doi.org/10.1038/nature04299
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04299
This article is cited by
-
Baf-mediated transcriptional regulation of teashirt is essential for the development of neural progenitor cell lineages
Experimental & Molecular Medicine (2024)
-
Cell-type-specific chromatin occupancy by the pioneer factor Zelda drives key developmental transitions in Drosophila
Nature Communications (2021)
-
Cancer stem cell fate determination: a nuclear phenomenon
The Nucleus (2019)
-
PAR3–PAR6–atypical PKC polarity complex proteins in neuronal polarization
Cellular and Molecular Life Sciences (2018)
-
Prefoldin and Pins synergistically regulate asymmetric division and suppress dedifferentiation
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.