Abstract
Atypical antipsychotic adjunctive therapy to lithium or valproate is effective in treating acute mania. Although continuation of atypical antipsychotic adjunctive therapy after mania remission reduces relapse of mood episodes, the optimal duration is unknown. As many atypical antipsychotics cause weight gain and metabolic syndrome, they should not be continued unless the benefits outweigh the risks. This 52-week double-blind placebo-controlled trial recruited patients with bipolar I disorder (n=159) who recently remitted from a manic episode during treatment with risperidone or olanzapine adjunctive therapy to lithium or valproate. Patients were randomized to one of three conditions: discontinuation of risperidone or olanzapine and substitution with placebo at (i) entry (‘0-weeks’ group) or (ii) at 24 weeks after entry (‘24-weeks’ group) or (iii) continuation of risperidone or olanzapine for the full duration of the study (‘52-weeks’ group). The primary outcome measure was time to relapse of any mood episode. Compared with the 0-weeks group, the time to any mood episode was significantly longer in the 24-weeks group (hazard ratio (HR) 0.53; 95% confidence interval (CI): 0.33, 0.86) and nearly so in the 52-weeks group (HR: 0.63; 95% CI: 0.39, 1.02). The relapse rate was similar in the 52-weeks group compared with the 24-weeks group (HR: 1.18; 95% CI: 0.71, 1.99); however, sub-group analysis showed discordant results between the two antipsychotics (HR: 0.48, 95% CI: 0.17; 1.32 olanzapine patients; HR: 1.85, 95% CI: 1.00, 3.41 risperidone patients). Average weight gain was 3.2 kg in the 52-weeks group compared with a weight loss of 0.2 kg in the 0-weeks and 0.1 kg in the 24-weeks groups. These findings suggest that risperidone or olanzapine adjunctive therapy for 24 weeks is beneficial but continuation of risperidone beyond this period does not reduce the risk of relapse. Whether continuation of olanzapine beyond this period reduces relapse risk remains unclear but the potential benefit needs to be weighed against an increased risk of weight gain.
Similar content being viewed by others
Introduction
Bipolar I disorder is a lifelong condition characterized by manic and depressive episodes. A significant proportion of patients experiencing an acute manic episode are treated with a combination of a mood stabilizer (that is, lithium or valproate) and an atypical antipsychotic.1, 2 There is general consensus as indicated by recommendations from various guidelines about continuing a mood stabilizer to reduce the risk of relapse or recurrence of a mood episode.3, 4, 5 In addition, there is also evidence from some6, 7, 8, 9 but not all10 studies that continuation of an atypical antipsychotic as an adjunct to a mood stabilizer provides additional benefit in reducing the risk of relapse but the optimal duration of such strategy remains unknown. For instance, different industry-funded registration trials have reported continuation of an atypical antipsychotic as being beneficial, compared with placebo, when given as adjunct for 6 months,9 12 months8 or 24 months.6, 7 However, none of these studies examined different durations for the atypical antipsychotic therapy within the same trial. An examination of survival curves from these trials suggests that most relapses in the placebo adjunctive therapy group occurred within the first 6 months of randomization. Does this mean that the atypical antipsychotic adjunctive therapy is beneficial mainly within the first 6 months of remission of an acute mood episode and not beyond? This is a critical question because atypical antipsychotics are associated with significant side effects such as weight gain, metabolic syndrome and extrapyramidal side effects and hence should not be continued unless their benefits beyond 6 months can be clearly demonstrated.
Therefore, the primary objective of this randomized double-blind placebo-controlled trial was to determine the efficacy of different durations (24 or 52 weeks) of atypical antipsychotic adjunctive therapy (that is, risperidone or olanzapine) to mood stabilizer vs discontinuing the atypical antipsychotic at study entry in preventing relapse of any mood episode in bipolar I disorder patients who recently remitted (within 6 weeks of remission) from an acute manic episode. An important additional objective was to investigate whether 52 weeks of treatment offered any additional benefit compared with discontinuing the atypical antipsychotic after 24 weeks.
Materials and methods
Subjects
Patients for the study were recruited from 17 Canadian Network for Mood and Anxiety Treatments (CANMAT)-affiliated academic centers in Canada and collaborating sites in Brazil. Study procedures were approved by the Research Ethics Boards of each site. A written informed consent was obtained from all patients after providing a complete description of the study. Patients aged ⩾17 years were eligible for the study if they were: (1) diagnosed with Bipolar I disorder; (2) treated within the previous 12 weeks, for a DSM-IV11 acute manic or mixed episode with a combination of mood stabilizer (lithium or valproate) and atypical antipsychotic (risperidone or olanzapine); (3) in remission from the manic or mixed episode for at least 2 weeks and no more than 6 weeks based on (i) a Clinical Global Impression Severity (CGI-S)12 score of 2 or less for 2 consecutive weeks or (ii) a Young Mania Rating Scale (YMRS)13 score of 8 or less and a Hamilton Rating Scale for Depression (HAM-D)14 21- item score of 8 or less for two consecutive weeks.
Patients with a history of comorbid substance abuse or other axis I disorders were allowed but those taking other psychotropic medication with the exception of benzodiazepines were excluded.
Trial design and interventions
This study was a multi-center three-parallel-group randomized double-blind placebo-controlled trial with up to 52 weeks of follow-up. After obtaining informed consent, patients were randomized to one of the three groups. Randomizations were stratified by drug combination and by center. The treating clinicians and all research personnel except the trial statistician and the pharmacist were blinded to treatment arm allocations. Patients randomized to the ‘0-weeks’ group tapered and discontinued risperidone or olanzapine over 2 weeks beginning on the day of randomization and received placebo substitution for the remaining 50 weeks. Patients randomized to the ‘24-weeks’ group received risperidone or olanzapine for 24 weeks. The antipsychotic was tapered and discontinued over the next 2 weeks with the placebo substitution for the remaining 26 weeks. Patients randomized to the ‘52-weeks’ group continued risperidone or olanzapine for 52 weeks. All patients continued the same mood stabilizer (lithium or valproate) they had been taking at study entry and serum levels were maintained within the therapeutic ranges (0.6–1.2 mmol/L for lithium and 350–830 μmol/L for valproate) throughout the 52 weeks. The dose and type of atypical antipsychotic were the same as the patient had been on at entry into the study. Allowed dosages for risperidone were 1–6 mg per day and olanzapine 5–25 mg per day. Patients were not allowed to receive any other psychotropic medication except benzodiazepines for sedation and anti-parkinsonian medication for extrapyramidal side effects. Patients were allowed to receive psychoeducation and counseling regarding sleep hygiene, healthy daily routines and rhythms, alcohol and substance abuse, anxiety management, conflict resolution and problem solving, as clinically indicated as part of their ongoing clinical care.
Study outcomes
Patients were assessed biweekly until week 8 and every 4 weeks thereafter up to week 52 by trained raters blind to treatment allocation using the following instruments: YMRS, HAMD-21, Montgomery-Asberg Depression Rating Scale (MADRS),15 CGI-S and Clinical Global Impression-Bipolar Severity (CGI-BP). Clinical Global Impression-Improvement (CGI-I) and CGI-BP change were assessed from week 2 onwards. Safety and tolerability were evaluated through clinical observation as well as using the Udvalg for Kliniske Undersøgelser Side Effect Rating Scale (UKU)16 as well as the Extrapyramidal Symptoms Rating Scale (ESRS)17 at baseline and at monthly visits. Laboratory measurements on blood sera were obtained at the screening visit and at weeks 12, 24, 36 and 52.
The primary outcome measure was the time to any mood episode, defined as any of the following events: (i) YMRS score of 15 or greater, (ii) HAM-D 21-item score of 15 or greater or HAM-D suicide item score of 3 or greater, (iii) CGI-S score of 3 or greater, (iv) hospitalization for treatment of mood symptoms or (v) suicide or suicide attempt. Patients experiencing a primary event were removed from further study follow-up.
Secondary outcome measures were time to a manic episode, time to a depressive episode and time to premature discontinuation from the study for any clinical reason (primary endpoint met, dose change in risperidone or olanzapine study medication, new intervention, adverse event). Primary outcome events were classified as manic or depressive based on the CGI-BP, YMRS and HAM-D scores.
Sample size
The planned sample size for the trial was 540 patients (180 per group). This sample size was based on two primary comparisons of the event proportions (24-weeks vs 0-weeks groups and 52-weeks vs 0-week groups) by 52 weeks, each at a two-sided significance level of 0.025 (to ensure an overall type I error rate less than 5%) with 80% power and allowing for a 25% drop-out rate. The assumed event proportions were 55% in the 0-week group and 38% (that is, absolute reduction of 17%) in each of the other two groups.
Statistical analysis
All analyses were conducted on an intent-to-treat basis and included all randomized patients. Kaplan–Meier cumulative incidence plots were used to summarize the time to any mood episode by treatment group. For the primary analysis, a Cox proportional hazards model with adjustment for the antipsychotic drug and for the mood stabilizer drug was used to compare the time to any mood episode across treatment groups. As a sensitivity analysis, a mixed effects (frailty) Cox model with site entered as a clustering variable was fit to account for potential site effects. Patients who did not experience the primary outcome were censored as of the time of last follow-up visit.
Similar analyses were used to evaluate the time to a manic episode, and the time to a depressive episode. When analyzing the time to a manic (depressive) event, patients experiencing a depressive event (manic event) were censored at the time of this event.
Changes in weight and laboratory parameters were calculated as the difference between the measurements at baseline and at last follow-up; if the measurement at last follow-up was missing, the most recently observed value was used.
Results
Patients and disposition
A total of 159 patients were randomized (52 to the 0-weeks group, 54 to the 24-weeks group and 53 to the 52-weeks group) into the trial across 17 sites. The recruitment for the study was much slower than anticipated and ultimately was stopped because of expiration of funding.
Patient characteristics were well-balanced across the groups except that the patients in the 0-weeks group had a higher percentage of women, lower mean weight and lower percentage with a history of or currently active psychiatric comorbidity compared with the patients in the other two groups, while the patients in the 24-weeks group had a lower percentage with alcohol/substance abuse (Table 1). The disposition of patients is summarized in Figure 1. A total of 34 (21%) patients discontinued the study (8, 14 and 12 patients in the 0-weeks, 24-weeks and 52-weeks groups, respectively) for reasons other than meeting the primary endpoint. The mean follow-up times were 18, 25 and 24 weeks for the 0-weeks, 24-weeks and 52-weeks groups, respectively.
Subject enrollment and disposition.
Primary outcomes
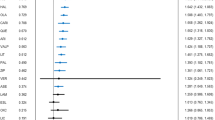
There were 39 primary events (depression=25, mania=14) among 52 patients in the 0-weeks group, 29 events (depression=23, mania=6) among 54 patients in the 24-weeks group and 29 (depression=22, mania=7) events among 53 patients in the 52-weeks group (Table 2). Kaplan–Meier cumulative incidence curves (Figure 2a) suggested that the time to any mood episode was longer in both 52-weeks and 24-weeks groups compared with the 0-weeks group. However, the time to any mood episode was similar between the 52-weeks and 24-weeks groups. In the adjusted Cox analysis, the hazard ratio (HR) for time to any mood episode was 0.53 for the 24-weeks group relative to the 0-weeks group (95% confidence interval (CI): 0.33, 0.86; P=0.01) and 0.63 for the 52-weeks group relative to the 0-weeks group (95% CI: 0.39, 1.02; P=0.06). The HR for the 52-weeks group relative to the 24-weeks group was 1.18 (95% CI: 0.71, 1.99; P=0.52). On the basis of the Kaplan–Meier curves, the estimated 52-week event rates were 65%, 65% and 87% in the 52-weeks, 24-weeks and 0-weeks groups, respectively. The results were negligibly different in the mixed effects Cox model accounting for clustering by site.
(a) Time to relapse of any mood episode. (b) Time to relapse of a manic episode. (c) Time to relapse of a depressive episode.
Secondary outcomes
Across groups, a total of 27 patients met criteria for a manic event and 70 for a depressive event. The time to a manic episode was longer in patients randomized to the 24-weeks and the 52-weeks groups compared with patients randomized to the 0-weeks group (Figure 2b) and statistically significant for the 24-weeks group (HR: 0.30, 95% CI: 0.12, 0.80; P=0.02) but not for the 52-weeks group (HR: 0.43, 95% CI: 0.17, 1.09; P=0.08). The time to a manic episode was shorter in the 52-weeks group compared with the 24-weeks groups but the difference was not statistically significant (HR: 1.41, 95% CI: 0.47, 4.25, P=0.54).
Although the time to a depressive episode was also longer in the 24-weeks and 52-weeks groups compared with the 0-weeks group (Figure 2c), this difference was not statistically significant for either group (HR for 24-weeks vs 0-weeks groups: 0.65; 95% CI: 0.37, 1.15, P=0.14: HR for 52-weeks vs 0-weeks groups: 0.73; 95% CI: 0.41, 1.29, P=0.28). In addition, the time to a depressive episode was similar in the 52-weeks group compared with the 24-weeks group (HR: 1.11; 95% CI: 0.62, 2.01, P=0.72).
The time to discontinuation for any clinical reason was longer in the 24-weeks and the 52-weeks groups compared with the 0-weeks group, but the effect was statistically significant only for the 24-weeks group (HR: 0.54, 95% CI: 0.34, 0.86, P=0.01) but not for the 52-weeks group (HR: 0.65, 95% CI: 0.41, 1.04, P=0.07). The time to discontinuation for any clinical reason was similar in the 52-weeks group compared with the 24-weeks group (HR: 1.22, 95% CI: 0.74, 1.99, P=0.44).
Subgroup analysis
In the olanzapine subgroup, the time to any mood episode was longer in both the 24-weeks and the 52-weeks groups compared with the 0-weeks group, with the difference reaching statistical significance for the 52-week group (HR: 0.23, 95% CI: 0.09, 0.59, P=0.003) but not for the 24-weeks group (HR: 0.47, 95% CI: 0.21, 1.07, P=0.07). The time to any mood episode was longer but not statistically significant in the 52-weeks group compared with the 24-weeks group (HR: 0.48, 95% CI: 0.17; 1.32, P=0.16).
In the risperidone subgroup, the time to any mood episode was longer in the 24-weeks group relative to 0-weeks group but was not statistically significant (HR: 0.57, 95% CI: 0.31, 1.05, P=0.07). Surprisingly, the time to any mood episode in the 52-week group was similar to that in the 0-weeks group (HR: 1.05, 95% CI: 0.59, 1.88, P=0.86) and shorter than in the 24-weeks group (HR: 1.85, 95% CI: 1.00, 3.41; P=0.05).
Adverse events
The only serious adverse event recorded was the single death from pneumonia, which occurred in the 52-weeks group, and was determined by the site investigator as being unrelated to the study medication. Rates of other adverse events were similar across the three groups (Table 3). The mean changes in the ESRS total score, Parkinsonism+Dystonia subscale and Dyskinesia subscale were similar across all three groups.
In terms of weight change, patients in the 52-weeks group gained significantly more weight (3.2 kg, P=0.01) compared with those in the 0-weeks group (lost 0.2 kg) and 24-weeks group (lost 0.1 kg). Within the olanzapine subgroup, the weight changes were a loss of 0.7 kg, a loss of 0.2 kg and a gain of 5.6 kg in the 0-week, 24-week and 52-week groups, respectively. The corresponding weight changes in the risperidone subgroup were a gain of 0.3 kg, no change and a gain of 1.3 kg. Clinically significant weight gain (⩾7% or more of baseline weight) was more common among patients in the 52-weeks group (25%) than in the 0-weeks (12%) and 24-weeks (15%) groups. In the olanzapine subgroup, more patients gained ⩾7% weight in the 52-weeks group (35%) compared with the 0-weeks group (5%) or the 24-weeks group (14%), while in the risperidone subgroup, these proportions were more similar across the groups (17%, 15% and 17% in the 0-weeks, 24-weeks and 52-weeks groups, respectively). Average change in glucose, cholesterol or triglycerides levels from baseline to last follow-up were similar in all three groups whether including all patients or in either antipsychotic subgroup.
Discussion
This is the first study to compare different durations of atypical antipsychotic adjunctive therapy in the maintenance treatment of bipolar I disorder after remission from an acute manic or mixed episode. The most important findings of the study are: (i) The time to relapse of any mood episode was significantly longer in the group that continued atypical antipsychotic adjunctive therapy for 24 weeks compared with the group that had their atypical antipsychotic discontinued at study entry; (ii) There was a trend for longer time to relapse of any mood episode in the 52-weeks group compared with the 0-weeks group; (iii) The time to relapse of any mood episode was similar in the 52-weeks group compared with the 24-weeks group; however, the sub-group analysis showed discordance in effects of risperidone and olanzapine beyond 24 weeks. In addition, weight gain was significantly greater and more patients gained ⩾7% body weight with 52 weeks of continued antipsychotic use compared with 24 weeks.
The findings of this study are highly clinically relevant and have immediate transformational value for the management of bipolar disorder. Although adjunctive atypical antipsychotic therapy was beneficial for 24 weeks after the remission of an acute manic episode, the benefits were not readily apparent beyond 24 weeks as overall, there were no differences in relapse rates between the 24-weeks and 52–weeks groups. Importantly, weight gain was significantly more common in the 52-weeks group compared with the other two groups. Thus, these data suggest that adjunctive atypical antipsychotic therapy beyond 24 weeks confers additional adverse event burden without necessarily offering definitive tangible clinical benefit in efficacy.
Although mania is the defining feature of bipolar I disorder, several studies suggest that depressive episodes and symptoms outnumber manic/hypomanic episodes/symptoms by a ratio of 3:1 during the course of bipolar disorder.18 Consistent with this, more patients in our study had depressive recurrences compared with manic recurrences (70 vs 27 events, respectively). Despite higher depressive recurrences, adjunctive therapy benefit was more apparent in preventing manic recurrences in the 24-weeks (HR: 0.30) and 52-weeks (HR: 0.43) groups compared with the 0-weeks group than for preventing depressive recurrences (HR: 0.65 for the 24 weeks group and HR: 0.73 for the 52 weeks group). These data are consistent with the clinical observation and findings from other studies which suggest that many atypical antipsychotics are more effective in preventing manic than depressive recurrences.8, 9, 19, 20
This study was not adequately powered for analysis of the antipsychotic subgroups, but several findings warrant discussion. The prophylactic effect of risperidone was apparent only for mania whereas for olanzapine, the benefit was seen mainly in preventing depression (Table 2). These findings are broadly consistent with previous studies21, 22 although the lack of effect of olanzapine in preventing mania is surprising. This might be because many of the patients in the olanzapine 0-weeks group had a depressive event, and thus left fewer patients at risk for mania. The HR for the time to any mood episode comparing the 24-weeks group with the 0-weeks group were similar for both olanzapine and risperidone. Hence, the finding of benefit of 24 weeks of therapy appears robust and applicable to both antipsychotics. In contrast, when comparing the 52-weeks group with the 24-weeks group, the results were discordant between the two antipsychotics. In the risperidone subgroup, the risk of relapse increased with a near doubling of the HR. Even if this result is a false signal and continued use of risperidone beyond 24 weeks does not truly increase the risk of relapse, it remains unlikely that any benefit exists. In the olanzapine subgroup, the risk of relapse decreased with the HR roughly halved though not statistically significant. This result could be because of inadequate sample size. However, this reduction in risk of relapse was accompanied by a 20% increase in risk of weight gain by at least 7% and an average weight gain of nearly 6 kg. Given that the weight gain is a predictor of poor clinical outcomes in bipolar disorder,23, 24 the potential clinical benefit of continuing olanzapine beyond 24 weeks needs to be balanced against the risks of weight-gain-associated morbidity and mortality.
The incidence of adverse events was relatively low and few patients dropped out of the study because of adverse events. There were no significant differences in rates of adverse events, metabolic parameters or ESRS scale scores between the three groups. This may be because of the enriched design in which only those patients who responded and tolerated these medications were eligible to enter the trial. Despite such enrichment in design, patients in the 52-weeks group gained considerably more weight and more patients in this group had clinically significant weight gain, and in particular, those who had received olanzapine adjunctive therapy. This suggests that weight gain with olanzapine may continue beyond 24 weeks.
This study has several strengths including the use of a stratified randomized double-blind design, that it recruited ‘real world’ patients with and without comorbidity, and allowed routine clinical management including psychoeducation and counseling as clinically indicated. However, some limitations must also be considered. First, this study included only risperidone and olanzapine; hence, the findings of this study may not be generalizable to all atypical antipsychotics. Nevertheless, the findings of this study are consistent with other maintenance studies showing that the efficacy in prevention of relapse is most apparent in the first 6 months. Second, owing to recruitment challenges, the final sample size was smaller than originally planned. Larger sample sizes would have yielded more precise assessments of the differences between the 24-weeks and 52-weeks groups in the olanzapine and risperidone subgroups and provided more definitive conclusions as to whether the discordant findings truly reflect a reduced risk of relapse with continued olanzapine use, an increased risk with continued risperidone use, or both. Third, comparisons of adverse event rates and change in outcome measures may be impacted by differences in the average length of follow-up across treatment groups. However, mean follow-up time in the 24-weeks and 52-weeks arms were similar and so do not explain the weight gain differences between these two groups. Last, clinicians were allowed to provide adjunctive psychological treatments as clinically appropriate. Although differences in types and duration of psychological treatments might have contributed to differences in efficacy, this is also a strength of this study in that it mirrored real world clinical practice.
In conclusion, the results of this study suggest that patients with bipolar I disorder who recently remitted from an acute manic episode with adjunctive risperidone or olanzapine therapy are less likely to have a recurrence if these medications are continued for 24 weeks vs discontinuing soon after remission of mania. However, benefits beyond 24 weeks are not apparent; subgroup results suggest continued risperidone use provides no benefit and although it remains unclear whether continued olanzapine use reduces the risk of recurrence, the potential benefit should be weighed against a concomitant increased risk of weight gain. Given the clinical significance of these findings, future studies should use the similar design to examine the applicability of these findings to other atypical antipsychotics that have less metabolic burden liability.
References
Miller DS, Yatham LN, Lam RW . Comparative efficacy of typical and atypical antipsychotics as add-on therapy to mood stabilizers in the treatment of acute mania. J Clin Psychiatry 2001; 62: 975–980.
Tohen M, Zarate CA Jr . Antipsychotic agents and bipolar disorder. J Clin Psychiatry 1998; 59: 138–148.
Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 2013; 15: 1–44.
Goodwin GM . Evidence-based guidelines for treating bipolar disorder: revised second edition—recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2009; 23: 346–388.
Grunze H, Vieta E, Goodwin GM, Bowden C, Licht RW, Moller HJ et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update 2012 on the long-term treatment of bipolar disorder. World J Biol Psychiatry 2013; 14: 154–219.
Vieta E, Suppes T, Eggens I, Persson I, Paulsson B, Brecher M . Efficacy and safety of quetiapine in combination with lithium or divalproex for maintenance of patients with bipolar I disorder (international trial 126). J Affect Disord 2008; 109: 251–263.
Suppes T, Vieta E, Liu S, Brecher M, Paulsson B . Maintenance treatment for patients with bipolar I disorder: results from a north american study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry 2009; 166: 476–488.
Marcus R, Khan A, Rollin L, Morris B, Timko K, Carson W et al. Efficacy of aripiprazole adjunctive to lithium or valproate in the long-term treatment of patients with bipolar I disorder with an inadequate response to lithium or valproate monotherapy: a multicenter, double-blind, randomized study. Bipolar Disord 2011; 13: 133–144.
Bowden CL, Vieta E, Ice KS, Schwartz JH, Wang PP, Versavel M . Ziprasidone plus a mood stabilizer in subjects with bipolar I disorder: a 6-month, randomized, placebo-controlled, double-blind trial. J Clin Psychiatry 2010; 71: 130–137.
Tohen M, Chengappa KNR, Suppes T, Baker RW, Zarate CA, Bowden CL et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry 2004; 184: 337–345.
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 1994.
Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W . Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res 1997; 73: 159–171.
Young RC, Biggs JT, Ziegler VE, Meyer DA . A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133: 429–435.
Hamilton M . A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62.
Montgomery SA, Asberg M . A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389.
Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K . The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 1987; 33: 41–100.
Chouinard G, Margolese HC . Manual for the Extrapyramidal Symptom Rating Scale (ESRS). Schizophr Res 2005; 76: 247–265.
Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 2002; 59: 530–537.
Quiroz JA, Yatham LN, Palumbo JM, Karcher K, Kushner S, Kusumakar V . Risperidone long-acting injectable monotherapy in the maintenance treatment of bipolar I disorder. Biol Psychiatry 2010; 68: 156–162.
Keck PE Jr., Calabrese JR, McQuade RD, Carson WH, Carlson BX, Rollin LM et al. A randomized, double-blind, placebo-controlled 26-week trial of aripiprazole in recently manic patients with bipolar I disorder. J Clin Psychiatry 2006; 67: 626–637.
Popovic D, Reinares M, Goikolea JM, Bonnin CM, Gonzalez-Pinto A, Vieta E . Polarity index of pharmacological agents used for maintenance treatment of bipolar disorder. Eur Neuropsychopharmacol 2012; 22: 339–346.
Vieta E, Montgomery S, Sulaiman AH, Cordoba R, Huberlant B, Martinez L et al. A randomized, double-blind, placebo-controlled trial to assess prevention of mood episodes with risperidone long-acting injectable in patients with bipolar I disorder. Eur Neuropsychopharmacol 2012; 22: 825–835.
Fagiolini A, Kupfer DJ, Houck PR, Novick DM, Frank E . Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry 2003; 160: 112–117.
Wildes JE, Marcus MD, Fagiolini A . Obesity in patients with bipolar disorder: a biopsychosocial-behavioral model. J Clin Psychiatry 2006; 67: 904–915.
Acknowledgements
This study was funded by a peer-reviewed grant to the investigators from the Canadian Institutes of Health Research. Lilly Pharmaceuticals supplied olanzapine/placebo study medication. Janssen Canada covered the costs for risperidone and identical placebo study medication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Lakshmi N Yatham reports grants from Lilly, Janssen and from the Canadian Institutes of Health Research during the conduct of the study. He also received grants and personal fees from AstraZeneca, Bristol Myers Squibb and Dianippon Sumitomo. He received personal fees from Forrest, Glaxo Smithkline, Lundbeck, Sunovion and Lilly outside the submitted work. Dr Serge Beaulieu reports grants and personal fees from Astra Zeneca, BMS, Lundbeck, Otsuka and Sunovion. He received grants from Novartis, Canadian Institutes of Health Research, Fonds de recherche du Québec, Réseau Santé Mentale au Québec, Brain and Behavior Research Foundation, Stanley Foundation and Pfizer Reseach Award. He reports receiving personal fees from Forest Laboratories, Lilly, Merck and Pfizer from outside the submitted work. Dr Ayal Schaffer reports grants from Canadian Institute of Health Research during the conduct of the study. He received grants from Pfizer Canada and personal fees from AstraZeneca, Eli Lilly, Sunovion, Lundbeck and Bristol-Myers Squibb outside the submitted work. Dr Marcia Kauer-Sant’Anna reports grants from Stanley Medical Research Institute, grants and personal fees from Eli-Lilly, and grants from CNPq-INCT-TM, CNPq-Universal, FIPE-HCPA and NARSAD outside the submitted work. Dr Flavio Kapzinski reports receiving support for research from AstraZeneca, Eli Lilly, Janssen-Cilag and Servier outside the submitted work. Dr Verinder Sharma reports grant support from Bristol-Myers Squibb, Cephalon, Elan Pharmaceuticals and Shire; personal fees from AstraZeneca, Eli Lilly, Janssen, Servier, Sunovion, Lundbeck and Bristol-Myers Squibb outside the submitted work. Dr Sagar V Parikh reports personal fees from Sunovion, Pfizer, BMS, Otsuka, Astra-Zeneca, Lundbeck and Lilly, and grant funding from Lundbeck outside the submitted work. Dr Andree Daigneault reports grants and personal fees from Astra Zeneca, BMS and personal fees from Lundbeck, Sunovion and Valeant, outside the submitted work. Dr David J Bond reports grants from Pfizer and personal fees from Sunovion, Pfizer, BMS, Otsuka, Astra-Zeneca and Janssen, outside the submitted work. Dr Roumen Milev reports grants and personal fees from Astra Zeneca, BMS, Pfizer and Eli Lilly. He also reports personal fees from Merck, Otsuka, Sunovion, Valeant, as well as personal fees and non-financial support from Lundbeck outside the submitted work. Dr Philippe Baruch reports personal fees from BMS and Sunovion, outside the submitted work. Dr Raymond W. Lam reports grants from Canadian Institutes of Health Research, other from Janssen and Eli Lilly, during the conduct of the study. He also received grants from Bristol Myers Squibb, Coast Capital Savings, Pfizer and St. Jude Medical; grants and personal fees from Lundbeck; and personal fees from AstraZeneca, Canadian Psychiatric Association, Lundbeck Institute, Otsuka, Servier, Canadian Network for Mood and Anxiety Treatments, outside the submitted work. In addition, Dr Lam has a patent Lam Employment Absence and Productivity Scale (LEAPS) issued, a patent Cambridge University Press with royalties paid, a patent Informa Press with royalties paid, and a patent Oxford University Press with royalties paid. Dr Beny Lafer, Dr Hong Qian, Dr Peter H Silverstone, Ms. Nazlin Walji, Dr Angelo da Cunha, Dr Joao Quevedo, Dr Rodrigo Dias, Dr Mauricio Kunz, Dr L Trevor Young and Dr Hubert Wong declare no conflict of interest.
PowerPoint slides
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Yatham, L., Beaulieu, S., Schaffer, A. et al. Optimal duration of risperidone or olanzapine adjunctive therapy to mood stabilizer following remission of a manic episode: A CANMAT randomized double-blind trial. Mol Psychiatry 21, 1050–1056 (2016). https://doi.org/10.1038/mp.2015.158
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.158
This article is cited by
-
A systematic review of interventions in the early course of bipolar disorder I or II: a report of the International Society for Bipolar Disorders Taskforce on early intervention
International Journal of Bipolar Disorders (2023)
-
Possible Pharmacodynamic and Pharmacokinetic Drug-Drug Interactions That Are Likely to Be Clinically Relevant and/or Frequent in Bipolar Disorder
Current Psychiatry Reports (2018)
-
Pharmacological Approaches to Minimizing Cardiometabolic Side Effects of Mood Stabilizing Medications
Current Treatment Options in Psychiatry (2017)