Abstract
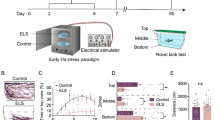
Upon binding of cortisol, the glucocorticoid receptor (GR) regulates the transcription of specific target genes, including those that encode the stress hormones corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone. Dysregulation of the stress axis is a hallmark of major depression in human patients. However, it is still unclear how glucocorticoid signaling is linked to affective disorders. We identified an adult-viable zebrafish mutant in which the negative feedback on the stress response is disrupted, due to abolition of all transcriptional activity of GR. As a consequence, cortisol is elevated, but unable to signal through GR. When placed into an unfamiliar aquarium (‘novel tank’), mutant fish become immobile (‘freeze’), show reduced exploratory behavior and do not habituate to this stressor upon repeated exposure. Addition of the antidepressant fluoxetine to the holding water and social interactions restore normal behavior, followed by a delayed correction of cortisol levels. Fluoxetine does not affect the overall transcription of CRH, the mineralocorticoid receptor (MR), the serotonin transporter (Serta) or GR itself. Fluoxetine, however, suppresses the stress-induced upregulation of MR and Serta in both wild-type fish and mutants. Our studies show a conserved, protective function of glucocorticoid signaling in the regulation of emotional behavior and reveal novel molecular aspects of how chronic stress impacts vertebrate brain physiology and behavior. Importantly, the zebrafish model opens up the possibility of high-throughput drug screens in search of new classes of antidepressants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dickmeis T . Glucocorticoids and the circadian clock. J Endocrinol 2009; 200: 3–22.
Lightman SL, Wiles CC, Atkinson HC, Henley DE, Russell GM, Leendertz JA et al. The significance of glucocorticoid pulsatility. Eur J Pharmacol 2008; 583: 255–262.
Pariante CM, Lightman SL . The HPA axis in major depression: classical theories and new developments. Trends Neurosci 2008; 31: 464–468.
Holsboer F . The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000; 23: 477–501.
Belmaker RH, Agam G . Mechanisms of disease: major depressive disorder. N Engl J Med 2008; 358: 55–68.
Carroll BJ . The Dexamethasone suppression test for melancholia. Br J Psychiatry 1982; 140: 292–304.
Chandler VL, Maler BA, Yamamoto KR . DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell 1983; 33: 489–499.
So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR . Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. Plos Genet 2007; 3: 927–938.
Meijsing SH, Pufall MA, So AY, Bates DL, Chen L, Yamamoto KR . DNA binding site sequence directs glucocorticoid receptor structure and activity. Science 2009; 324: 407–410.
Surjit M, Ganti KP, Mukherji A, Ye T, Hua G, Metzger D et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 2011; 145: 224–241.
de Kloet ER, Joels M, Holsboer F . Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005; 6: 463–475.
Sahay A, Hen R . Hippocampal neurogenesis and depression. Novartis Found Symp 2008; 289: 152–160.
Binder EB, Nemeroff CB . The CRF system, stress, depression and anxiety – insights from human genetic studies. Mol Psychiatry 2010; 15: 574–588.
Muto A, Orger MB, Wehman AM, Smear MC, Kay JN, Page-McCaw PS et al. Forward genetic analysis of visual behavior in zebrafish. Plos Genet 2005; 1: 575–588.
Schaaf MJM, Chatzopoulou A, Spaink HP . The zebrafish as a model system for glucocorticoid receptor research. Comp Biochem Physiol A Mol Integr Physiol 2009; 153: 75–82.
Danielsen M, Northrop JP, Ringold GM . The mouse glucocorticoid receptor - mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J 1986; 5: 2513–2522.
Giguere V, Hollenberg SM, Rosenfeld MG, Evans RM . Functional domains of the human glucocorticoid receptor. Cell 1986; 46: 645–652.
Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 1998; 93: 531–541.
Kassel O, Herrlich P . Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol 2007; 275: 13–29.
Riml S, Schmidt S, Ausserlechner MJ, Geley S, Kofler R . Glucocorticoid receptor heterozygosity combined with lack of receptor auto-induction causes glucocorticoid resistance in Jurkat acute lymphoblastic leukemia cells. Cell Death Differ 2004; 11: S65–S72.
Luisi BF, Xu WX, Otwinowski Z, Freedman LP, Yammamoto KR, Siegler PB . Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature 1991; 352: 497–505.
Bernier NJ, Lin XW, Peter RE . Differential expression of corticotropin-releasing factor (CRF) and urotensin I precursor genes, and evidence of CRF gene expression regulated by cortisol in goldfish brain. Gen Comp Endocrinol 1999; 116: 461–477.
Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S et al. Analyzing habituation responses to novelty in zebrafish (Danio rerio). Behav Brain Res 2010; 208: 450–457.
Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouvela Jr A . Measuring anxiety in zebrafish: a critical review. Behav Brain Res 2010; 214: 157–171.
Engeszer RE, Ryan MJ, Parichy DM . Learned social preference in zebrafish. Curr Biol 2004; 14: 881–884.
Kikusui T, Winslow JT, Mori Y . Social buffering: relief from stress and anxiety. Philos Trans Royal Soc B Biol Sci 2006; 361: 2215–2228.
Ruiz M, Lind U, Gafvels M, Eggertsen G, Carlstedt-Duke J, Nilsson L et al. Characterization of two novel mutations in the glucocorticoid receptor gene in patients with primary cortisol resistance. Clin Endocrinol (Oxf) 2001; 55: 363–371.
Charmandari E, Kino T, Ichijo T, Zachman K, Alatsatianos A, Chrousos GP . Functional characterization of the natural human glucocorticoid receptor (hGR) mutants hGR alpha R477H and hGR alpha G679S associated with generalized glucocorticoid resistance. J Clin Endocrinol Metab 2006; 91: 1535–1543.
Marcelli M, Zoppi S, Grino PB, Griffin JE, Wilson JD, McPhaul MJ . A mutation in the DNA-binding domain of the androgen receptor gene causes complete testicular feminization in a patient with receptor-positive androgen resistance. J Clin Invest 1991; 87: 1123–1126.
Champagne DL, Hoefnagels CCM, de Kloet RE, Richardson MK . Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav Brain Res 2010; 214: 332–342.
Blaser RE, Chadwick L, McGinnis GC . Behavioral measures of anxiety in zebrafish (Danio rerio). Behav Brain Res 2010; 208: 56–62.
Maximino C, de Brito TM, Colmanetti R, Pontes AA, de Castro HM, de Lacerda RI et al. Parametric analyses of anxiety in zebrafish scototaxis. Behav Brain Res 2010; 210: 1–7.
Lockwood B, Bjerke S, Kobayashi K, Guo S . Acute effects of alcohol on larval zebrafish: a genetic system for large-scale screening. Pharmacol Biochem Behav 2004; 77: 647–654.
Grossman L, Utterback E, Stewart A, Gaikwad S, Chung KM, Suciu C et al. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behav Brain Res 2010; 214: 277–284.
Ressler KJ, Nemeroff CB . Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety 2000; 12: 2–19.
Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M . Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev 2008; 32: 1174–1184.
Clements S, Moore FL, Schreck CB . Evidence that acute serotonergic activation potentiates the locomotor-stimulating effects of corticotropin-releasing hormone in juvenile chinook salmon (Oncorhynchus tshawytscha). Horm Behav 2003; 43: 214–221.
Carpenter RE, Watt MJ, Forster GL, Øverli Ø, Bockholt C, Renner KJ et al. Corticotropin releasing factor induces anxiogenic locomotion in trout and alters serotonergic and dopaminergic activity. Horm Behav 2007; 52: 600–611.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–809.
Chourbaji S, Gass P . Glucocorticoid receptor transgenic mice as models for depression. Brain Res Rev 2008; 57: 554–560.
Kolber BJ, Wieczorek L, Muglia LJ . Hypothalamic-pituitary-adrenal axis dysregulation and behavioral analysis of mouse mutants with altered glucocorticoid or mineralocorticoid receptor function. Stress 2008; 11: 321–338.
Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci 2005; 25: 6243–6250.
Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA 2005; 102: 473–478.
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 1999; 23: 99–103.
Montkowski A, Barden N, Wotjak C, Stec I, Ganster J, Meaney M et al. Long-term antidepressant treatment reduces behavioral deficits in transgenic mice with impaired glucocorticoid receptor function. J Neuroendocrinol 1995; 7: 841–845.
Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA 2004; 101: 11851–11856.
Rozeboom AM, Akil H, Seasholtz AF . Mineralocorticoid receptor overexpression in forebrain decreases anxiety-like behavior and alters the stress response in mice. Proc Natl Acad Sci USA 2007; 104: 4688–4693.
Groeneweg FL, Karst H, de Kloet ER, Joels M . Rapid non-genomic effects of corticosteroids and their role in the central stress response. J Endocrinol 2011; 209: 153–167.
Di S, Malcher-Lopes R, Halmos KC, Tasker JG . Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 2003; 23: 4850–4857.
Groc L, Choquet D, Chaouloff F . The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci 2008; 11: 868–870.
Liu L, Wang CN, Ni X, Sun JH . A rapid inhibition of NMDA receptor current by corticosterone in cultured hippocampal neurons. Neurosci Lett 2007; 420: 245–250.
Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 2010; 327: 348–351.
Acknowledgements
We thank J Kitamoto and M Suzawa for technical assistance on some of our preliminary experiments; E Gahtan, NM Shah, R Carpenter, R Fletterick, R Fernald and M Dallman for advice and comments; S Hong and I Dawid for sending us the kohtalo mutant for complementation testing; G Rechavi for support of LZ, and members of the Baier lab for discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Ziv, L., Muto, A., Schoonheim, P. et al. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol Psychiatry 18, 681–691 (2013). https://doi.org/10.1038/mp.2012.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2012.64
Keywords
This article is cited by
-
Glucocorticoid receptor activation reduces food intake independent of hyperglycemia in zebrafish
Scientific Reports (2022)
-
Genomic polymorphisms at the crhr2 locus improve feed conversion efficiency through alleviation of hypothalamus-pituitary-interrenal axis activity in gibel carp (Carassius gibelio)
Science China Life Sciences (2022)
-
Genome-Wide Association and Expression Analysis Revealed the Candidate Variants and Molecular Underpinnings of Cold-Stress Response in Large Yellow Croaker
Marine Biotechnology (2022)
-
Neural substrates involved in the cognitive information processing in teleost fish
Animal Cognition (2021)
-
Ethanol affects behavior and HPA axis activity during development in zebrafish larvae
Scientific Reports (2020)