Abstract
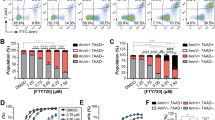
Lysosome membrane permeabilization (LMP) mediates cell death in a variety of cancer cells. However, little is known about lysosomes and LMP in chronic lymphocytic leukemia (CLL). Owing to drug resistance and toxicity in CLL patients, better treatment strategies are required. Our results show that CLL cells were sensitive to the lysosomotropic agent siramesine. Furthermore, this drug was more effective in CLL cells, regardless of prognostic factors, compared with normal B cells. Siramesine caused LMP, lipid peroxidation and transcription factor EB nuclear translocation followed by mitochondrial membrane potential loss and reactive oxygen species release. Siramesine-induced cell death was blocked by lipid antioxidants, but not by soluble antioxidants or protease inhibitors. To determine whether CLL cells had altered lysosomes, we investigated sphingolipid metabolism as the lysosome is a hub for lipid metabolism. We found that CLL cells had more lysosomes, increased sphingosine-1-phosphate phosphatase 1 (SPP1) expression, and increased levels of sphingosine compared with normal B cells. Raising sphingosine levels increased LMP and cell death in CLL cells, but not in normal B cells. Together, these results show that excess sphingosine in CLL cells could contribute to their sensitivity toward LMP. Thus, targeting the lysosome could be a novel therapeutic strategy in CLL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–1174.
Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005; 23: 4079–4088.
Jain P, Keating M, Wierda W, Estrov Z, Ferrajoli A, Jain N et al. Outcomes of patients with chronic lymphocytic leukemia (CLL) after discontinuing ibrutinib. Blood 2015; 125: 2062–2067.
Morabito F, Gentile M, Seymour JF, Polliack A . Ibrutinib idelalisib and obinutuzumab for the treatment of patients with chronic lymphocytic leukemia: three new arrows aiming at the target. Leuk Lymphoma 2015; 56: 1–20.
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370: 1101–1110.
Cartron G, de Guibert S, Dilhuydy M-S, Morschhauser F, Leblond V, Dupuis J et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood 2014; 124: 2196–2202.
Jak M, van Bochove GG, Reits EA, Kallemeijn WW, Tromp JM, Umana P et al. CD40 stimulation sensitizes CLL cells to lysosomal cell death induction by type II anti-CD20 mAb GA101. Blood 2011; 118: 5178–5188.
Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood 2011; 117: 4519–4529.
Fehrenbacher N, Bastholm L, Kirkegaard-Sørensen T, Rafn B, Bøttzauw T, Nielsen C et al. Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-associated membrane proteins 1 and 2. Cancer Res 2008; 68: 6623–6633.
Ostenfeld MS, Fehrenbacher N, Høyer-Hansen M, Thomsen C, Farkas T, Jäättelä M . Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Res 2005; 65: 8975–8983.
Erdal H, Berndtsson M, Castro J, Brunk U, Shoshan MC, Linder S . Induction of lysosomal membrane permeabilization by compounds that activate p53-independent apoptosis. Proc Natl Acad Sci USA 2005; 102: 192–197.
Mena S, Rodríguez ML, Ponsoda X, Estrela JM, Jäättela M, Ortega AL . Pterostilbene-induced tumor cytotoxicity: a lysosomal membrane permeabilization-dependent mechanism. PLoS One 2012; 7: e44524.
Sukhai MA, Prabha S, Hurren R, Rutledge AC, Lee AY, Sriskanthadevan S et al. Lysosomal disruption preferentially targets acute myeloid leukemia cells and progenitors. J Clin Invest 2013; 123: 315–328.
Mijatovic T, Mathieu V, Gaussin J-F, De Nève N, Ribaucour F, Van Quaquebeke E et al. Cardenolide-induced lysosomal membrane permeabilization demonstrates therapeutic benefits in experimental human non-small cell lung cancers. Neoplasia 2006; 8: 402–412.
Don AS, Lim XY, Couttas TA . Re-configuration of sphingolipid metabolism by oncogenic transformation. Biomolecules 2014; 4: 315–353.
Petersen NH, Olsen OD, Groth-Pedersen L, Ellegaard A-M, Bilgin M, Redmer S et al. Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell 2013; 24: 379–393.
Zheng Y, Wen J, Nguyen J, Cachia MA, Wang C, Sun Y . Decreased deformability of lymphocytes in chronic lymphocytic leukemia. Sci Rep 2015; 5: 7613.
Goñi FM, Alonso A . Effects of ceramide and other simple sphingolipids on membrane lateral structure. Biochim Biophys Acta 2009; 1788: 169–177.
Breslow DK, Weissman JS . Membranes in balance: mechanisms of sphingolipid homeostasis. Mol Cell 2010; 40: 267–279.
Nowakowski GS, Hoyer JD, Shanafelt TD, Zent CS, Call TG, Bone ND et al. Percentage of smudge cells on routine blood smear predicts survival in chronic lymphocytic leukemia. J Clin Oncol 2009; 27: 1844–1849.
Woyach JA, Johnson AJ, Byrd JC . The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood 2012; 120: 1175–1184.
Brown DA, London E . Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 2000; 275: 17221–17224.
Abramoff M, Magalhaes P, Ram S . Image processing with ImageJ. Biophotonics Int 2004; 11: 36–42.
Fehrenbacher N, Jäättelä M . Lysosomes as targets for cancer therapy. Cancer Res 2005; 65: 2993–2995.
Ostenfeld MS, Høyer-Hansen M, Bastholm L, Fehrenbacher N, Olsen OD, Groth-Pedersen L et al. Anti-cancer agent siramesine is a lysosomotropic detergent that induces cytoprotective autophagosome accumulation. Autophagy 2008; 4: 487–499.
Boya P, Andreau K, Poncet D, Zamzami N, Perfettini J-L, Metivier D et al. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med 2003; 197: 1323–1334.
Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA et al. A gene network regulating lysosomal biogenesis and function. Science 2009; 325: 473–477.
Česen MH, Repnik U, Turk V, Turk B . Siramesine triggers cell death through destabilisation of mitochondria, but not lysosomes. Cell Death Dis 2013; 4: e818.
Aits S, Jäättelä M . Lysosomal cell death at a glance. J Cell Sci 2013; 126: 1905–1912.
Yoon J-Y, Szwajcer D, Ishdorj G, Benjaminson P, Xiao W, Kumar R et al. Synergistic apoptotic response between valproic acid and fludarabine in chronic lymphocytic leukaemia (CLL) cells involves the lysosomal protease cathepsin B. Blood Cancer J 2013; 3: e153.
Settembre C, Ballabio A . Lysosome: regulator of lipid degradation pathways. Trends Cell Biol 2014; 24: 743–750.
Hamer I, Van Beersel G, Arnould T, Jadot M . Lipids and lysosomes. Curr Drug Metab 2012; 13: 1371–1387.
Pralhada Rao R, Vaidyanathan N, Rengasamy M, Mammen Oommen A, Somaiya N, Jagannath MR . Sphingolipid metabolic pathway: an overview of major roles played in human diseases. J Lipids 2013; 2013: 178910.
Gutierrez A, Tschumper RC, Wu X, Shanafelt TD, Eckel-Passow J, Huddleston PM et al. LEF-1 is a prosurvival factor in chronic lymphocytic leukemia and is expressed in the preleukemic state of monoclonal B-cell lymphocytosis. Blood 2010; 116: 2975–2983.
Heading C . Siramesine H Lundbeck. Curr Opin Investig Drugs 2001; 2: 266–270.
Hollister LE . Plasma concentrations of tricyclic antidepressants in clinical practice. J Clin Psychiatry 1982; 43: 66–69.
Meyerhoff A, Albrecht R, Meyer JM, Dionne P, Higgins K, Murphy D . US Food and Drug Administration approval of ciprofloxacin hydrochloride for management of postexposure inhalational anthrax. Clin Infect Dis 2004; 39: 303–308.
Kayden HJ, Hatam L, Traber MG, Conklyn M, Liebes LF, Silber R . Reduced tocopherol content of B cells from patients with chronic lymphocytic leukemia. Blood 1984; 63: 213–215.
Farber CM, Liebes LF, Kanganis DN, Silber R . Human B lymphocytes show greater susceptibility to H2O2 toxicity than T lymphocytes. J Immunol 1984; 132: 2543–2546.
Vianello A, Casolo V, Petrussa E, Peresson C, Patui S, Bertolini A et al. The mitochondrial permeability transition pore (PTP) - an example of multiple molecular exaptation? Biochim Biophys Acta 2012; 1817: 2072–2086.
Brenner C, Moulin M . Physiological roles of the permeability transition pore. Circ Res 2012; 111: 1237–1247.
Zoratti M, Szab I . Electrophysiology of the inner mitochondrial membrane. J Bioenerg Biomembr 1994; 26: 543–553.
Jitschin R, Hofmann AD, Bruns H, Giessl A, Bricks J, Berger J et al. Mitochondrial metabolism contributes to oxidative stress and reveals therapeutic targets in chronic lymphocytic leukemia. Blood 2014; 123: 2663–2672.
Carew JS, Nawrocki ST, Xu RH, Dunner K, McConkey DJ, Wierda WG et al. Increased mitochondrial biogenesis in primary leukemia cells: the role of endogenous nitric oxide and impact on sensitivity to fludarabine. Leukemia 2004; 18: 1934–1940.
Ryland LK, Fox TE, Liu X, Loughran TP, Kester M . Dysregulation of sphingolipid metabolism in cancer. Cancer Biol Ther 2011; 11: 138–149.
Chen M-B, Yang L, Lu P-H, Fu X-L, Zhang Y, Zhu Y-Q et al. MicroRNA-101 down-regulates sphingosine kinase 1 in colorectal cancer cells. Biochem Biophys Res Commun 2015; 463: 954–960.
Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J 2006; 20: 386–388.
Le Scolan E, Pchejetski D, Banno Y, Denis N, Mayeux P, Vainchenker W et al. Overexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood 2005; 106: 1808–1816.
Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S . Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res 2002; 281: 115–127.
Nava VE, Cuvillier O, Edsall LC, Kimura K, Milstien S, Gelmann EP et al. Sphingosine enhances apoptosis of radiation-resistant prostate cancer cells. Cancer Res 2000; 60: 4468–4474.
Ullio C, Casas J, Brunk UT, Sala G, Fabriàs G, Ghidoni R et al. Sphingosine mediates TNFα-induced lysosomal membrane permeabilization and ensuing programmed cell death in hepatoma cells. J Lipid Res 2012; 53: 1134–1143.
Kågedal K, Zhao M, Svensson I, Brunk UT . Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem J 2001; 359: 335–343.
Acknowledgements
This work was supported by grants from the Canadian Cancer Society Research Institute and the CancerCare Manitoba Foundation. First author is supported by a PhD fellowship from Research Manitoba. Dr Gibson is a Margaret A. Seller Chair in Cell Biology. We acknowledge the contributions from the Manitoba Tumor Bank in the processing and banking of CLL patient samples.
Author contributions
Dielschneider and Eisenstat performed experiments and analyzed results. Mi and Curtis performed HPLC analysis of sphingosine. Work by Dielschneider and Xiao contributed to the funding for this project. Johnston and Gibson provided invaluable experimental design and editorial advice. Dielschneider and Gibson wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Dielschneider, R., Eisenstat, H., Mi, S. et al. Lysosomotropic agents selectively target chronic lymphocytic leukemia cells due to altered sphingolipid metabolism. Leukemia 30, 1290–1300 (2016). https://doi.org/10.1038/leu.2016.4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2016.4
This article is cited by
-
Octyl syringate is preferentially cytotoxic to cancer cells via lysosomal membrane permeabilization and autophagic flux inhibition
Cell Biology and Toxicology (2023)
-
Organelle-targeted therapies: a comprehensive review on system design for enabling precision oncology
Signal Transduction and Targeted Therapy (2022)
-
FV-429 induces autophagy blockage and lysosome-dependent cell death of T-cell malignancies via lysosomal dysregulation
Cell Death & Disease (2021)
-
Cationic amphiphilic drugs induce elevation in lysoglycerophospholipid levels and cell death in leukemia cells
Metabolomics (2020)
-
Targeting sphingolipid metabolism as an approach for combination therapies in haematological malignancies
Cell Death Discovery (2018)