Abstract
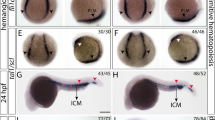
Human oncogenes involved in the development of hematological malignancies have been widely used to model experimental leukemia. However, models of myeloid leukemia rarely reproduce the human disease in full, due to genetic complexity or to difficulties in targeting leukemia initiating cells. Here, we used a zebrafish genetic model to induce the expression of oncogenic RAS in endothelial cells, including the hemogenic endothelium of the dorsal aorta that generates hematopoietic cells, and observed the development of a myelo-erythroid proliferative disorder. In larvae, the phenotype is characterized by disruption of the vascular system and prominent expansion of the caudal hematopoietic tissue. In few surviving juveniles, increased number of immature hematopoietic cells and arrest of myeloid maturation was found in kidney marrow. Peripheral blood showed increased erythroblasts and myeloid progenitors. We found that the abnormal phenotype is associated with a downregulation of the Notch pathway, whereas overexpressing an activated form of Notch together with the oncogene prevents the expansion of the myelo-erythroid compartment. This study identifies the downregulation of the Notch pathway following an oncogenic event in the hemogenic endothelium as an important step in the pathogenesis of myelo-erythroid disorders and describes a number of potential effectors of this transformation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hirschi KK . Hemogenic endothelium during development and beyond. Blood 2012; 119: 4823–4827.
Medvinsky A, Rybtsov S, Taoudi S . Embryonic origin of the adult hematopoietic system: advances and questions. Development 2011; 138: 1017–1031.
Kissa K, Herbomel P . Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 2010; 464: 112–115.
Bertrand JY, Kim AD, Violette EP, Stachura DL, Cisson JL, Traver D . Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development 2007; 134: 4147–4156.
Chen AT, Zon LI . Zebrafish blood stem cells. J Cell Biochem 2009; 108: 35–42.
Bertrand JY, Cisson JL, Stachura DL, Traver D . Notch signaling distinguishes 2 waves of definitive hematopoiesis in the zebrafish embryo. Blood 2010; 115: 2777–2783.
Martin CS, Moriyama A, Zon LI . Hematopoietic stem cells, hematopoiesis and disease: lessons from the zebrafish model. Genome Med 2011; 3: 83.
McGrath KE, Palis J . Hematopoiesis in the yolk sac: more than meets the eye. Exp Hematol 2005; 33: 1021–1028.
Le X, Langenau DM, Keefe MD, Kutok JL, Neuberg DS, Zon LI . Heat shock-inducible Cre/Lox approaches to induce diverse types of tumors and hyperplasia in transgenic zebrafish. Proc Natl Acad Sci USA 2007; 104: 9410–9415.
Yeh JR, Munson KM, Elagib KE, Goldfarb AN, Sweetser DA, Peterson RT . Discovering chemical modifiers of oncogene-regulated hematopoietic differentiation. Nat Chem Biol 2009; 5: 236–243.
Bolli N, Payne EM, Grabher C, Lee JS, Johnston AB, Falini B et al. Expression of the cytoplasmic NPM1 mutant (NPMc+) causes the expansion of hematopoietic cells in zebrafish. Blood 2010; 115: 3329–3340.
Forrester AM, Grabher C, McBride ER, Boyd ER, Vigerstad MH, Edgar A et al. NUP98-HOXA9-transgenic zebrafish develop a myeloproliferative neoplasm and provide new insight into mechanisms of myeloid leukaemogenesis. Br J Haematol 2011; 155: 167–181.
Payne E, Look T . Zebrafish modelling of leukaemias. Br J Haematol 2009; 146: 247–256.
Jing L, Zon LI . Zebrafish as a model for normal and malignant hematopoiesis. Dis Model Mech 2011; 4: 433–438.
Forrester AM, Berman JN, Payne EM . Myelopoiesis and myeloid leukaemogenesis in the zebrafish. Adv Hematol 2012; 2012: 358518.
Teittinen KJ, Gronroos T, Parikka M, Ramet M, Lohi O . The zebrafish as a tool in leukemia research. Leuk Res 2012; 36: 1082–1088.
Hart A, Melet F, Grossfeld P, Chien K, Jones C, Tunnacliffe A et al. Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity 2000; 13: 167–177.
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF . Stages of embryonic development of the zebrafish. Dev Dyn 1995; 203: 253–310.
Herwig L, Blum Y, Krudewig A, Ellertsdottir E, Lenard A, Belting HG et al. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol 2011; 21: 1942–1948.
Santoriello C, Anelli V, Alghisi E, Mione M . Highly penetrant melanoma in a zebrafish model is independent of ErbB3b signaling. Pigment Cell Melanoma Res 2012; 25: 287–289.
Scheer N, Campos-Ortega JA . Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech Dev 1999; 80: 153–158.
Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N et al. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev 2009; 126: 898–912.
Bertrand JY, Kim AD, Teng S, Traver D . CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development 2008; 135: 1853–1862.
Kawakami K . Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol 2004; 77: 201–222.
Redd MJ, Kelly G, Dunn G, Way M, Martin P . Imaging macrophage chemotaxis in vivo: studies of microtubule function in zebrafish wound inflammation. Cell Motil Cytoskeleton 2006; 63: 415–422.
Haferlach T, Kohlmann A, Wieczorek L, Basso G, Kronnie GT, Bene MC et al. Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: report from the International Microarray Innovations in Leukemia Study Group. J Clin Oncol 2010; 28: 2529–2537.
Asakawa K, Suster ML, Mizusawa K, Nagayoshi S, Kotani T, Urasaki A et al. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci USA 2008; 105: 1255–1260.
Lawson ND, Weinstein BM . in vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 2002; 248: 307–318.
Distel M, Wullimann MF, Koster RW . Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc Natl Acad Sci USA 2009; 106: 13365–13370.
Lawson ND, Vogel AM, Weinstein BM . sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 2002; 3: 127–136.
Santoriello C, Gennaro E, Anelli V, Distel M, Kelly A, Koster RW et al. Kita driven expression of oncogenic HRAS leads to early onset and highly penetrant melanoma in zebrafish. PLoS One 2010; 5: e15170.
Pase L, Layton JE, Kloosterman WP, Carradice D, Waterhouse PM, Lieschke GJ . miR-451 regulates zebrafish erythroid maturation in vivo via its target gata2. Blood 2009; 113: 1794–1804.
Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D . Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 2010; 464: 108–111.
Vardiman JW . The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: an overview with emphasis on the myeloid neoplasms. Chem Biol Interact 2010; 184: 16–20.
Guruharsha KG, Kankel MW, Artavanis-Tsakonas S . The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat Rev Genet 2012; 13: 654–666.
Kato H, Taniguchi Y, Kurooka H, Minoguchi S, Sakai T, Nomura-Okazaki S et al. Involvement of RBP-J in biological functions of mouse Notch1 and its derivatives. Development 1997; 124: 4133–4141.
Siekmann AF, Lawson ND . Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 2007; 445: 781–784.
Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 2011; 9: 541–552.
Lancrin C, Sroczynska P, Serrano AG, Gandillet A, Ferreras C, Kouskoff V et al. Blood cell generation from the hemangioblast. J Mol Med (Berl) 2010; 88: 167–172.
Clements WK, Kim AD, Ong KG, Moore JC, Lawson ND, Traver D . A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature 2011; 474: 220–224.
Zhang C, Patient R, Liu F . Hematopoietic stem cell development and regulatory signaling in zebrafish. Biochim Biophys Acta 2013; 1830: 2370–2374.
Liu F, Walmsley M, Rodaway A, Patient R . Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol 2008; 18: 1234–1240.
Acknowledgements
We are grateful to D Traver, K Kawakami, R Koester, M Tada, M Affolter, N Lawson, G Lieschke, L Zon and member of his lab, for plasmids, transgenic lines and suggestions in the course of this project. ZF Screen for carrying out the deep sequencing. EA was supported by a COST-STSM-BM0804-040712-019714 travel fellowship; MD by a postdoctoral fellowship of the German Academic Exchange Service (DAAD) and an EMBO fellowship. EA, MM and DR were partially supported by BCC Pompiano e Franciacorta and Lions Bassa Bresciana Foundation. MCM was supported by the Helmholtz research programme BioInterfaces.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
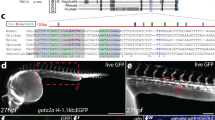
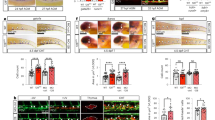
E Alghisi and MC Mione conceived the study, carried out most of the experimental work and analysis, and wrote the paper. M Distel carried out the experiments shown in Figure 4, in the supplementary movies and in Supplementary Figure S3, provided interpretation and analysis of the data and contributed to writing the manuscript. M Malagola contributed to cytological analysis and to the writing of the Introduction. V Anelli and C Santoriello contributed to the experiments shown in Figures 2, 6 and 7. L Herwig and A Krudewig generated the transgenic line tg(fli1ep:GAL4FF)ubs3, C Henkel performed the analysis of the deep sequencing results and contributed to Figure 7d. Russo provided insights into the interpretation of the data. All authors commented on the ms.
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Alghisi, E., Distel, M., Malagola, M. et al. Targeting oncogene expression to endothelial cells induces proliferation of the myelo-erythroid lineage by repressing the notch pathway. Leukemia 27, 2229–2241 (2013). https://doi.org/10.1038/leu.2013.132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.132
Keywords
This article is cited by
-
Zebrafish as a model for leukemia and other hematopoietic disorders
Journal of Hematology & Oncology (2015)
-
Danio rerio: Small Fish Making a Big Splash in Leukemia
Current Pathobiology Reports (2014)