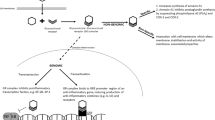
Abstract
Objective:
To compare asthma history and pulmonary function in adolescents born prematurely with very low birth weight with and without antenatal steroid exposure.
Study Design:
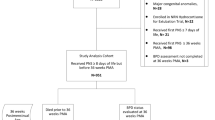
We studied 188 fourteen-year olds (94 exposed, 84 male). We used parent report to ascertain asthma and asthma-related symptoms and spirometry to assess pulmonary function. Steroid-exposed and -unexposed groups were compared using Mann–Whitney U-tests (continuous variables), χ2 analysis (categorical variables) and logistic regression (multivariate analyses).
Result:
The steroid-exposed group had greater prevalence of larger airway obstruction (35% vs 21%), and steroid-exposed adolescents with birth weights <1000 g had 4.5-fold higher odds of larger airway obstruction. Wheezing in the past 12 months was two times as prevalent in steroid-exposed adolescents with birth weights between 1000 and 1500 g.
Conclusion:
Antenatal steroid exposure does not provide long-term benefits for pulmonary outcomes in adolescents born prematurely with very low birth weight in the era of surfactant therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Hamilton BE, Martin JA, Ventura SJ . Births: Preliminary Data for 2010. National Center for Health Statistics: Hyattsville, MD, USA, 2011.
Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR et al. Trends in neonatal morbidity and mortality for very low birth weight infants. Am J Obstet Gynecol 2007; 196 (147): e1–e8.
ACOG Committee on Obstetric Practice. ACOG Committee Opinion No. 475: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol 2011; 117: 422–424.
Roberts D, Dalziel S . Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2006; 3: CD004454.
Doyle LW, Ford GW, Rickards AL, Kelly EA, Davis NM, Callanan C et al. Antenatal corticosteroids and outcome at 14 years of age in children with birth weight less than 1501 grams. Pediatrics 2000; 106: E2.
Dalziel SR, Rea HH, Walker NK, Parag V, Mantell C, Rodgers A et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax 2006; 61: 678–683.
Wiebicke W, Poynter A, Chernick V . Normal lung growth following antenatal dexamethasone treatment for respiratory distress syndrome. Pediatr Pulmonol 1988; 5: 27–30.
Smolders-de Haas H, Neuvel J, Schmand B, Treffers PE, Koppe JG, Hoeks J . Physical development and medical history of children who were treated antenatally with corticosteroids to prevent respiratory distress syndrome: a 10- to 12-year follow-up. Pediatrics 1990; 86: 65–70.
Wong YC, Beardsmore CS, Silverman M . Antenatal dexamethasone and subsequent lung growth. Arch Dis Child 1982; 57: 536–538.
Philip AG . Neonatal mortality rate: is further improvement possible? J Pediatr 1995; 126: 427–433.
Horbar JD, Wright EC, Onstad L . Decreasing mortality associated with the introduction of surfactant therapy: an observational study of neonates weighing 601 to 1300 grams at birth. The Members of the National Institute of Child Health and Human Development Neonatal Research Network. Pediatrics 1993; 92: 191–196.
Palta M, Sadek-Badawi M, Sheehy M, Albanese A, Weinstein M, McGuinness G et al. Respiratory symptoms at age 8 years in a cohort of very low birth weight children. Am J Epidemiol 2001; 154: 521–529.
Hung Y-L, Hsieh W-S, Chou H-C, Yang Y-H, Chen C-Y, Tsao P-N . Antenatal steroid treatment reduces childhood asthma risk in very low birth weight infants without bronchopulmonary dysplasia. J Perinat Med 2010; 38: 95–102.
Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R et al. CDC growth charts: United States. Adv Data 2000; 314: 1–27.
Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8: 483–491.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338.
Hankinson JL, Odencrantz JR, Fedan KB . Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159: 179–187.
Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 2000; 161: 309–329.
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968.
Blumenthal MN . Genetic, epigenetic, and environmental factors in asthma and allergy. Ann Allergy Asthma Immunol 2012; 108: 69–73.
Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM . Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988; 82: 527–532.
Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA 1995; 273: 413–418.
Lu FL, Hsieh C-J, Caffrey JL, Lin M-H, Lin Y-S, Lin C-C et al. Body mass index may modify asthma prevalence among low-birth-weight children. Am J Epidemiol 2012; 176: 32–42.
Vyas J, Kotecha S . Effects of antenatal and postnatal corticosteroids on the preterm lung. Arch Dis Child Fetal Neonatal Ed 1997; 77: F147–F150.
Narayanan M, Owers-Bradley J, Beardsmore CS, Mada M, Ball I, Garipov R et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med 2012; 185: 186–191.
Doyle LW, Ford GW, Davis NM, Callanan C . Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci 2000; 98: 137–142.
Finken MJJ, Meulenbelt I, Dekker FW, Frolich M, Walther FJ, Romijn JA et al. Abdominal fat accumulation in adults born preterm exposed antenatally to maternal glucocorticoid treatment is dependent on glucocorticoid receptor gene variation. J Clin Endocrinol Metab 2011; 96: E1650–E1655.
Kelly BA, Lewandowski AJ, Worton SA, Davis EF, Lazdam M, Francis J et al. Antenatal glucocorticoid exposure and long-term alterations in aortic function and glucose metabolism. Pediatrics 2012; 129: e1282–e1290.
Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet 2005; 365: 1856–1862.
Acknowledgements
We acknowledge Ms Alice Scott, RN, for her hard work in recruiting participants and coordinating the project, Ms Patty Brown, RN, for her assistance in recruiting participants, the personnel of the Clinical Research Unit for their support with data collection and processing, and the participants and their families without whom this research would not be possible. This research was funded by NICHD PO1 HD047584, the Clinical Research Unit of Wake Forest Baptist Medical Center Grant MO1-RR07122, Forsyth Medical Center and the Wake Forest School of Medicine Department of Pediatric Research Funds. The sponsors had no involvement in the design, collection, analysis or interpretation of data, the writing of the manuscript or the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nixon, P., Washburn, L. & O'Shea, T. Antenatal steroid exposure and pulmonary outcomes in adolescents born with very low birth weight. J Perinatol 33, 806–810 (2013). https://doi.org/10.1038/jp.2013.69
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2013.69
Keywords
This article is cited by
-
Antenatal corticosteroids and cardiometabolic outcomes in adolescents born with very low birth weight
Pediatric Research (2017)