Abstract
Objective:
To identify the risk factors of HIV vertical transmission in pregnant women.
Study Design:
Observational cohort study. Between 2002 and 2003, 479 HIV-infected pregnant women in a PMTCT (prevention of the mother-to-child transmission) program were followed up with their infants at delivery, until 15 months with infant HIV testing.
Results:
Of these 281 infants had a definitive HIV result by 15 months of age, and 31.7% of the infants become HIV infected. In univariate analysis the risk factor identified were presence of vaginal discharge, genital itchiness, genital ulcers, dysuria, abnormal breast and vaginal infections (Trichomonas, Bacteria vaginosis and Candida) in the mother at enrolment. In multivariate analysis vaginal infections risk ratio (RR) 1.72(1.03–2.88) and abnormal breast RR 4.36(2.89–6.58) were predictors of HIV vertical transmission.
Conclusion:
There is need to screen for vaginal infections (Trichomonas, Bacteria vaginosis and Candida) and examine pregnant women for mastitis to identify women at risk of HIV vertical transmission for prevention.
Similar content being viewed by others
Introduction
In resource-limited settings, the rate of mother-to-child transmission of human immunodeficiency virus (HIV) infection can reach as high as 40%, particularly with prolonged breastfeeding.1 The majority (90%) occur in sub-Saharan Africa.2 Current approaches to reduce this transmission involve antiretroviral drugs given during pregnancy, at delivery and postnatally to the infant. In poor communities, short-course regimens based on single-dose nevirapine to the mother and infant are being used.3
In 2007, Zimbabwe had about 7 million people in the reproductive age (15 to 49 years) with a total fertility rate of 3.3 children per woman.4 Estimated adult HIV prevalence was 15% and only 29% of HIV-infected pregnant women received antiretroviral prophylaxis as part of the Prevention of the mother-to-child transmission (PMTCT) of HIV national program.4 The single-dose nevirapine regimen was being used in the national program at this time. The estimated HIV prevalence in pregnant women was 25.7% in 2002, and although it has dropped to 17.7% in 2006, the prevalence remains high.5 With such a high prevalence of HIV and limited access to antiretroviral prophylaxis, it becomes important to identify risk factors of mother-to-child transmission of HIV in this setting where prevention is still a problem and early infant diagnosis is still a challenge. Factors that have been identified in other settings might not be relevant in this resource-poor setting, and therefore an assessment of various risk factors of HIV vertical transmission was performed among a cohort of women and infants who participated in a PMTCT program in Zimbabwe.
Methods
Setting
The study was conducted at three primary maternal and child health clinics in peri-urban areas around the capital city of Harare, (namely Epworth, Seke North and St Mary's) in Zimbabwe.
Design
This was a prospective observational cohort study of mother and child pairs followed up for 15 months. Between April 2002 and November 2003 HIV-infected pregnant women were enrolled into the study from 36 weeks of gestation, after obtaining informed consent. Pre- and post-test HIV counseling was offered as part of the national PMTCT program. Baseline information included sociodemographic characteristics, medical history of sexually transmitted infections, gynecological examination findings, vaginal swabs and blood sampling for full blood counts and serology for sexually transmitted infections, namely syphilis and herpes simplex type-2. Mothers were to receive 200 mg nevirapine in labor and their infants were to be given nevirapine 2 mg kg−1 body weight within 72 h of delivery according to the national guidelines (HIVNET 012).3
Follow-up
From delivery, mother and child pairs were followed up until 15 months post-delivery. This was an overall breastfeeding cohort of infants in whom breastfeeding patterns were documented. In addition to infant blood sampling for HIV testing at delivery, 6 weeks, 4 months, 9 months and 15 months, infants were assessed for signs and symptoms of AIDS by a pediatrician. The time points coincided with the infant vaccination schedule in Zimbabwe. Feeding history was obtained from the caregivers. All HIV-exposed infants were provided with cotrimoxazole prophylaxis until their definitive HIV status was known. This was done mainly to prevent Pneumocystis carinii pneumonia, which is a major cause of early mortality among HIV-infected infants.6 All HIV-infected infants were referred to the appropriate pediatric HIV/AIDS clinics for care according to the national guidelines. At this time, antiretroviral drugs were not available in the public sector.
Laboratory methods
Wet mount preparations were used under microscopy for diagnosis of Trichomonas vaginalis, Candida albicans and Bacteria vaginosis. Syphilis was diagnosed with Rapid Reagin test and confirmed with Tissue Plasminogen Hemagglutination Assay (Randox Laboratories, Crumlin, County Antrim, UK). Herpes simplex type-2 was diagnosed with an Enzyme-linked Immunosorbent Assay (Focus Diagnostics, Cypress, CA, USA). Infant blood samples were processed and tested for HIV with DNA PCR (Roche Diagnostics, Indianapolis, IN, USA) if the infants were less than 15 months of age, and with rapid HIV antibody tests, Determine (Abbott Diagnostics, Abbott Park, IL, USA) and Oraquick (Abbott Diagnostics) if 15 months or older. Both the Determine and Oraquick tests were performed for every sample taken at 15 months or older, and HIV DNA PCR was used as a tie-breaker. A time interval of 15 months was selected as the provisional exit for the study while waiting for the 18-month routine vaccination visit for further confirmation of infant HIV results and missed exit visits.7
Statistical analysis
Baseline descriptions of maternal sociodemographic characteristics were compared between mothers who transmitted HIV and those who did not. χ2-tests and relative risks for mother-to-child transmission were calculated for sexually transmitted infections, infant characteristics and maternal baseline full blood counts. Thereafter, factors that had a P-value of less than 0.25 in the univariate analysis were included in multivariate analysis to control for confounding and to identify the most important risk factors for HIV vertical transmission. The multivariate models were run separately for maternal and infant risk factors.
Results
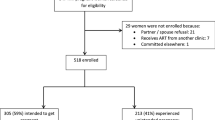
From the initial cohort of 479 HIV-infected pregnant women at enrolment, 45 (9.4%) had no delivery information. Of the 434 remaining mother and infant pairs, 46 (10.6%) infants died without any definitive HIV test result. A total of 281 (65%) had a definitive HIV result by 15 months of age. Of these, 89 (31.7%) infants became HIV infected by 15 months of age (Figure 1).
There were no significant differences between the mothers who transmitted HIV and those who did not transmit in terms of maternal and partner's age, gravidity, marital status, partner circumcision status and risky behaviors, such as any maternal alcohol consumption and vaginal herb use (Table 1). Table 2 considers some factors in the maternal WHO clinical staging of AIDS. During the study period, HIV viral loads and CD4 counts were not available in the public sector, and therefore the total lymphocyte count is mentioned. There were no significant differences between the maternal total lymphocyte count, hemoglobin, presence of axillary and/or cervical lymphadenopathy, history of prolonged fever and diarrhea and presence of tuberculosis in the past 5 years between HIV transmitters and non-transmitters. Mothers whose breasts were abnormal at physical examination (swollen and tender, discharging pus), a possible indicator of mastitis at enrolment, were twice as likely to be HIV vertical transmitters, 61.5 vs 30.0% (risk ratio 2.07 (1.28 to 3.32). Maternal death during the 15-month follow-up period was a predictor of vertical transmission (Table 2).
There were significant differences between the HIV vertical transmitters and the non-transmitters on the presence of abnormal vaginal discharge, genital itchiness, genital ulcers and dysuria (Table 3). However, physical examination confirmation of genital ulcer disease was not statistically different. When the types of abnormal vaginal discharge were considered individually, that is, yellow, gray and curd-like, there were no statistical differences between the two groups. The confirmed presence of vaginal infections, namely, T. vaginalis, B. vaginosis and C. albicans in the mother at enrolment, was associated with HIV vertical transmission (Table 3) and it remained a risk after multivariate analysis of all maternal factors with a risk ratio of 1.72 (1.03 to 2.88).
When we examined infant characteristics by HIV status, low birth weight infants and those who subsequently died during follow-up were more likely to be HIV infected (Table 4). Breastfeeding was not a statistically significant risk factor for being HIV infected. The median duration of exclusive breastfeeding was low in these two groups and was not significantly different between the transmitters (3 months (interquartile range 2; 6) and non-transmitters (4 months (interquartile range 2; 6). Forty percent of mother–infant pairs who did not receive nevirapine transmitted HIV compared with 30% of those who received nevirapine. We controlled for potential confounding with a multivariate analysis of risk factors of HIV vertical transmission (Table 5). After controlling for other maternal factors (signs and symptoms of sexually transmitted infections, confirmed vaginal infections and proxy maternal disease stage as indicated by maternal mortality during the study period), mothers with abnormal breasts at enrolment were four times more likely to transmit HIV (risk ratio 4.16 (1.10 to 15.9).
Discussion
Our finding that vaginal infections in HIV-infected pregnant women in the last trimester are a risk of HIV vertical transmission is important. The infections, namely, C. albicans, T. vaginalis and B. vaginosis, double the risk. There is paucity of data on these findings, but in a study by Fawzi et al.,2 gonorrhea was found to be related to intrauterine transmission. A possible biological explanation is that these infections can be associated with chorioamnionitis, increased viral shedding in secretions and inflammatory cytokine production, which facilitate HIV transmission.8 This study was performed at low-risk peripheral clinics where all women delivered vaginally. An increased risk of perinatal HIV transmission among herpes simplex virus type-2 seropositive women and an increased risk of intrapartum HIV transmission among women shedding herpes simplex type-2 were reported by Bollen et al.9
Mastitis has been reported to increase the risk of breastfeeding transmission.10, 11, 12 There is paucity of information on antenatal mastitis in HIV-infected women. In our study, the presence of abnormal breasts at about 36 weeks' gestation, which was a proxy for mastitis, was associated with a four times risk of HIV vertical transmission. Breastfeeding infants at 6 weeks of age were three times more likely to be HIV infected after multivariate analysis, although this was not statistically significant with a wide confidence interval. The trend of the adjusted risk ratios becomes more precise by 9 months of age. When feeding replacements are affordable and the practice is feasible, alternative foods are preferred to breast milk. The women were counseled to stop breastfeeding at 6 months, if feasible, but a significant proportion continued breastfeeding up to 9 months and beyond. Breastfeeding is a major health-promoting factor for infants and children in developing countries, but the risk of MTCT of HIV by this route is challenging traditional practices and health policies in low resource countries.13 Exclusive breastfeeding has been found to reduce transmission relative to mixed feeding.14 In our study, the median duration of exclusive breastfeeding in HIV-infected infants was only 3 months after our mothers were counseled about the benefits of exclusive breast feeding.
Deaths of infants and mothers during follow-up were associated with infant HIV infection. Maternal death implies advanced AIDS and higher viral loads, which we were unable to measure because of resource constraints at the time of the study. It is known that HIV-infected infants are at increased risk of mortality particularly in the first year of life, and hence the need to offer them early treatment.15
In a study by Bobat et al.,16 low hemoglobin during pregnancy defined by hemoglobin of less than 10 g per 100 ml was associated with an increased risk of HIV vertical transmission. This finding was not evident in our study. In the same study, a positive syphilis serology was not associated with vertical transmission.16 Our finding, which grouped positive serology for herpes simplex type-2 and syphilis because of the few cases of positive syphilis serology, had similar results.
Low birth weight has been found to be associated with in utero HIV transmission.17 Our low birth weight was unlikely to be influenced by prematurity because recruitment of the mothers occurred from 36 weeks' gestational age. However, with the control of breastfeeding and nevirapine intake, it dropped from statistical significance.
Despite our findings of increased vertical HIV transmission associated with maternal genital infections, breastfeeding and mastitis, our study is not without limitations. First, this study was not a randomized trial but a prospective cohort, and thus any of the findings may be inaccurate because of confounding bias. Further, we did not have complete follow-up of the women and infants in the study, which may lead to bias as well. For some of the predictors of HIV transmission that we examined, we were inadequately powered, particularly with few non-breastfed infants and few mother and child pairs who did not receive nevirapine. This study cannot be generalized to other settings without similar breastfeeding practices and a high prevalence of HIV similar to ours.
In conclusion, in resource-limited communities with HIV-infected pregnant women who opt to breastfeed, there is a need to consider vaginal infection screening, particularly T. vaginalis, B. vaginosis and C. albicans, and examination of women for possible mastitis to identify women at risk of HIV vertical transmission. This enables them to have early treatment, which could protect the infant. Small-for-dates HIV-infected pregnant women and low birth weight infants need special attention and early management. Early infant diagnosis and management of HIV-infected infants are priorities to reduce mortality.
References
Newell M . Current Issues in the prevention of mother-to-child transmission of HIV-1 infection. Trans R Soc Trop Med Hyg 2006; 100 (1): 1–5.
Fawzi W, Msamanga G, Renjifo B, Spieegelman D, Urassa E, Hashemi L et al. Predictors of intrauterine and intrapartum transmission of HIV-1 among Tanzanian women. AIDS 2001; 15 (9): 1–9.
Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C . Intrapartum and neonatal single-dose nevirapine compared with Zidovudine for prevention of mother to child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999; 354: 795–802.
UNAIDS 2008, http://www.who.int/hiv18/2/2009.
United Nationations General Assembly (UNGASS) Report on HIV and AIDS. Zimbabwe country report January 2006-December 2007.
Gill CJ, Sabin LL, Tham J, Hamer DH . Reconsidering empirical cotrimoxazole prophylaxis for infants exposed to HIV infection. Bull World Health Organ 2004; 82 (4): 290–298.
Palasanthiran P, Robertson P, Graham G, Hughes C, Ziegler JB . Decay of transplacental human immunodeficiency virus type-1 (HIV-1) antibodies in neonates and infants. Annu Conf Australas Soc HIV Med 1994; 6: 165 (abstract).
Taha E, Gray RH . Genital tract infections and perinatal transmission of HIV. Ann NY Acad Sci 2000; 918: 84–98 (abstract).
Bollen LJ, Whitehead SJ, Mock PA, Leelawiwat W, Asavapiriyanont S, Chalermchockchareonkit A et al. Maternal herpes simplex virus type 2 co-infection increases the risk of perinatal HIV transmission: possibility to further decrease transmission? AIDS 2008; 22 (10): 1169–1176.
Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet 2003; 362: 859–868.
Semba RD . Mastitis and transmission of human immunodeficiency virus through breast milk. Ann NY Acad Sci 2006; 918: 156–162.
Embree JE, Njenga S, Datta P, Nagelkerke NJ, Ndinya-Achola JO, Mohammed Z et al. Risk factors for postnatal mother-child transmission of HIV-1. AIDS 2000; 14 (16): 2535–2541.
Ogundele MO, Coulter JB . HIV transmission through breastfeeding: problems and prevention. Ann Trop Paediatri 2003; 23 (2): 91–106.
Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML et al. Mother to child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet 2007; 369 (957): 1107–1116.
Zijenah L, Moulton L, Illif P, Natthoo K, Munjoma M, Mutasa M et al. Timing of mother to child transmission of HIV -1 and infant mortality in the first 6 months of life in Harare, Zimbabwe. AIDS 2004; 18 (2): 273–280.
Bobat R, Coovadia H, Coutsoudis A, Moodley D . Determinants of mother to child transmission of HIV-1 infection in a cohort from Durban, South Africa. Pediatr Infect Dis 1996; 15 (7): 604–610.
Magder LS, Mofenson L, Paul ME, Zorrilla CD, Blattner WA, Tuomala RE et al. Risk factors for in utero and intrapartum transmission of HIV. JAIDS 2005; 38 (1): 87–95.
Acknowledgements
We gratefully acknowledge the women who participated in this study as well as the study staff. Special mention goes to the LETTEN Foundation and Professor Letten F Saugstad for funding the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Gumbo, F., Duri, K., Kandawasvika, G. et al. Risk factors of HIV vertical transmission in a cohort of women under a PMTCT program at three peri-urban clinics in a resource-poor setting. J Perinatol 30, 717–723 (2010). https://doi.org/10.1038/jp.2010.31
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2010.31
Keywords
This article is cited by
-
Current status of medication adherence and infant follow up in the prevention of mother to child HIV transmission programme in Addis Ababa: a cohort study
Journal of the International AIDS Society (2011)
-
Preventing mother-to-child transmission of HIV in urban Zimbabwe
Journal of Perinatology (2010)
-
Preventing mother-to-child HIV transmission in Zimbabwe
Journal of Perinatology (2010)