Abstract
Three amino acid-derived compounds, haenamindole (1) and 2'-epi-fumiquinazolines C (2) and D (3), were isolated from cultures of a fungicolous isolate of Penicillium lanosum (MYC-1813=NRRL 66231). Compound 1 was also encountered in cultures of P. corylophilum (MYC-418=NRRL 28126). Structure elucidation of these metabolites was based mainly on high resolution mass spectrometry and NMR data analysis. Haenamindole (1) was found to be a recently reported diketopiperazine-type metabolite that incorporates an unusual β-Phe unit. Analysis of X-ray crystallographic data and the products of acid hydrolysis of 1 enabled a conclusive, slightly modified stereochemical assignment for haenamindole. Fumiquinazoline analog 2 is a new natural product, while related compound 3 has been previously reported only as a product of an in vitro enzymatic step and of a genetically engineered fungal culture. Compounds 1 and 3 showed antiinsectan activity against the fall armyworm Spodoptera frugiperda.
Similar content being viewed by others
Introduction
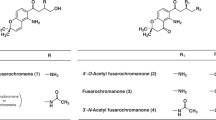
Although Penicillium spp. are well-known as prolific producers of bioactive natural products, many members of the genus still remain to be chemically investigated. Our studies of mycoparasitic and fungicolous fungi have afforded a variety of antiinsectan metabolites, including decaturins and thiersinines obtained from fungicolous isolates of previously undescribed Penicillium spp.1, 2 During our continued studies of such fungi, rice fermentation cultures of a fungicolous isolate of Penicillium lanosum (MYC-1813=NRRL 66231) provided an organic extract showing antiinsectan activity towards the fall armyworm Spodoptera frugiperda. Citrinin has been implicated in the toxicity of grain contaminated by this fungus,3, 4 and a chemotaxonomic study of P. lanosum strains resulted in detection of other known compounds, such as cycloaspeptide A, griseofulvin and sclerotigenin.5 Chemical investigation of the P. lanosum MYC-1813 extract led to the isolation of three amino acid-derived metabolites (Figure 1), including the recently reported haenamindole (1)6 and a new fumiquinazoline analog, 2'-epi-fumiquinazoline C (2). A third metabolite obtained, 2'-epi-fumiquinazoline D (3), had been reported only as a product of in vitro enzymatic semisynthesis, and of a genetically engineered culture of Penicillium aethiopicum.7 X-ray crystallographic analysis of 1 enabled an unambiguous stereochemical assignment, resulting in a revision relative to that originally proposed on the basis of NMR data alone. Details of these results are presented here.
Results and discussion
An ethyl acetate extract of P. lanosum cultures was fractionated by silica gel column chromatography, affording compound 1 as a major constituent. Subsequent application of another fraction to reversed-phase HPLC afforded samples of compounds 1–3 and sclerotigenin.8 The molecular formula of 1 was established as C29H28N4O5 (18 degrees of unsaturation) on the basis of high resolution mass spectrometry and NMR data. Signals accounting for all 28 protons (four exchangeable) and 29 carbons were observed in the 1H, 13C and DEPT NMR spectra (Table 1), and these data revealed the aromatic amino acid-derived nature of 1. Three aromatic subunits, including two phenyl groups and one ortho-disubstituted benzenoid ring, were recognized by analysis of 1H and 13C NMR and HMBC data (Table 1). These data were reminiscent of those expected for citreoindole (4),9 initially suggesting a simple derivative thereof. However, further analysis of these data and 2D NMR correlations allowed the extension of the two phenyl units to phenylalanine (Phe) and β-phenylalanine (β-Phe) substructures (summarized in Supplementary Figure S1 of the Supplementary Information), while 4 contains two equivalents of Phe.9 Methine H-12 showed COSY correlations with diastereotopic methylene H2-13 and NH-11. HMBC correlations from H-12 to phenyl group carbons C-14, C-15/19 and C-13, and from H2-13 to carbonyl carbon C-1, C-12 and only one of the carbons of the second phenyl group (C-14), located CH-12 β- to the carbonyl and established the β-Phe unit. β-Phe is an uncommon unit among fungal metabolites; however, a compound named haenamindole was recently reported from a marine-derived Penicillium sp. and assigned a structure comprised of β-Phe, modified Trp and diketopiperazine units.6 Comparison of 1H NMR data for 1 in DMSO-d6 with those reported for haenamindole revealed that the two compounds are identical, as all signals were within 0.01–0.02 p.p.m. of the literature values, and the coupling constants also matched very well. The connectivity was independently confirmed by analysis of COSY and HMBC data (Supplementary Figure S1). In addition, the presence of an N–OH group was verified by a positive FeCl3 test,10 and treatment of 1 with TMSCHN2 afforded a monomethylation product.
The relative configuration of the diketopiperazine unit was proposed on the basis of a weak, unresolved, but discernible long-range 5JHH coupling (<1 Hz) detected between H-21 and H-24 in the COSY spectrum, suggesting a cis relationship according to literature reports describing analogous observation of 5JHH coupling in similar systems.11, 12 Analysis of NOESY data enabled assignments of the relative configuration in the tricyclic ring system comprised of the β-Phe and Trp units. A NOESY correlation of H-10 with H-12 revealed that these hydrogens are cis to each other, and adoption of axial or pseudoaxial orientations was supported by the presence of a large JH12-H13 value (11 Hz). The signal for H-20b correlated with the 9-OH, while H-20a correlated with H-10, placing the 9-OH and H-10 on opposite faces of the molecule. The resulting stereochemical assignments, however, could not be related to the distal diketopiperazine unit with these data. Therefore, efforts to obtain a crystal were undertaken, ultimately resulting in determination of the relative configuration of 1 by X-ray crystallography as shown in Figure 2. The absolute configuration of 1 was determined based on the identification of l-Phe by both chiral TLC and HPLC analysis of the acid hydrolyzate. Notably, despite a literature report to the contrary,13 d- and l-β-Phe failed to resolve under either set of experimental conditions. Although both d- and l-β-Phe have been encountered from natural sources other than fungi,14, 15, 16, 17, 18, 19, 20, 21 to our knowledge, the only other fungal metabolite containing an l-β-Phe unit is the cyclic peptide cyclochlorotine, produced by Penicillium islandicum.22, 23
The above data reveal that the configuration at C-21 of structure 1 is opposite to that originally proposed for haenamindole. The literature proposal was based on the absence of a ROESY correlation between the two α-protons (H-21 and H-24) of the diketopiperazine unit.6 The two samples appear to represent the same compound, as they afford virtually identical NMR data and close specific rotation values. Upon request, the authors of the original work kindly provided an authentic sample for direct comparison. A ca. 1:1 mixture of the two samples displayed only one set of 1H NMR signals, confirming that 1 is haenamindole. Thus, we propose here revision of the stereochemical structure of haenamindole to that shown in 1.
1H NMR data for 3 include signals typical of amino acid units, as was the case for 1, but other signals present were indicative of significant structural modification. Aromatic 1H and 13C NMR shift values for 3 showed close resemblance to those of tryptoquivaline- and fumiquinazoline-type compounds, which contain quinazolinone and other aromatic subunits and afford UV data similar to those of 3.24, 25 In particular, the molecular formula of 3 (C24H21N5O4) assigned based on HRESIMS data matched that of fumiquinzolines C and D. Further investigation revealed that 1H and 13C NMR shift values for 3 (Table 2) were identical to those reported for 2'-epi-fumiquinazoline D, a product generated by the treatment of 2'-epi-fumiquinazoline A with Aspergillus fumigatus flavoenzyme Af12070.7 This compound was also detected as a product of a genetically modified culture of P. aethiopicum.7 Interestingly, evidence for formation of 2'-epi-fumiquinazoline C (2) was sought in these same studies, but this product was not detected, in contrast to the results of a similar experiment in which both fumiquinazolines C and D were produced from fumiquinazoline A.7 However, because 1H NMR J-values and multiplicities were not provided in the literature report, the structure of 3 was independently confirmed by analysis of HMBC and NOESY data, and characterization data are reported here.

Compound 2 was recognized as a more polar isomer of 3 based on HRESIMS data, leading to the use of acetone-d6 for initial NMR data analysis (Table 2). 1H NMR data for 2 in CDCl3 were also collected, and these matched closely with those reported for fumiquinazoline C, except for the values at positions C-2', C-11' and C-13'.25 Such a discrepancy is consistent with the kind of difference observed in the 1H NMR data between fumiquinazoline D and 2'-epi-fumiquinazoline D (3), suggesting that 2 is the 2'-epimer of fumiquinazoline C. A NOESY correlation (see below) of H-15 to H-2' rather than to H3-13', as is the case in fumiquinazoline C, supported the proposal of stereochemical alteration at position 2'.25 The planar structure of 2 was confirmed to be identical with that of fumiquinazoline C by analysis of 13C NMR and HMBC data as shown in Figure 3. In particular, comparison of the 13C NMR shift value at position C-3 (δC 81.8) with that of fumiquinazolines C (δC 84.2) and D (δC 70.8) clearly indicated oxygenation of non-protonated sp3 carbon C-3.25 Therefore, the oxygen atom at C-3 was connected to C-3' to establish an ether linkage and verify the gross structure of 2.
The relative configuration of the imidazoindolone ring system was determined by analysis of NOESY data (Figure 3). Correlations of H-2' with H3-13' and H2-15 placed these hydrogens on the same face of the ring, while the opposite orientation was assigned for H-2' in fumiquinazoline C. The configurations at C-3 and C-14 of 2 were presumed to match those of fumiquinazoline C due to their very similar 1H and 13C NMR data. A correlation was observed between H-7 and H3-13' in the NOESY data for 3, but not in the data for 2. This difference is consistent with the expected, significantly greater distance between the two positions calculated for the most stable conformer of 2 (Spartan '10). Therefore, 2 was concluded to be 2'-epi-fumiquinazoline C. This stereochemical difference was further supported by observation of an opposite-sign cotton effect in the ECD spectrum near 240–250 nm relative to data reported for fumiquinazoline C. A similar change in cotton effect sign was observed for 3 relative to the data for fumiquinazoline D. The 3′-epimer of 2 was also reported as an intermediate synthetic product of fumiquinazoline H.26 The structures of fumiquinazolines display various levels of complexity, and the biosynthetic route associated with these triptoquivaline-type compounds has been of interest in genetic studies for identification of the function corresponding to each gene cluster.7, 27
The isolate of P. lanosum used in this work was obtained from the surface of a wood decay fungus collected in Hawaii. Similar studies of another fungicolous Penicillium isolate, in this case P. corylophilum (MYC-418=NRRL 28126), also afforded compound 1. MYC-418 was isolated from a sclerotium of Aspergillus flavus that had been buried in the Illinois River Valley Sand Field. The closest known relative of 1 is citreoindole (4), a moderately cytotoxic metabolite (IC50 vs HeLa cells=8.4 μg ml−1) that has been reported only once before, from a hybrid strain derived from cell fusion experiments with P. citreo-viride protoplasts.9 The structure of 4 differs from that of 1 by replacement of l-β-Phe with l-Phe and by the absence of the N-hydroxyl group.
Haenamindole (1) and 2'-epi-fumiquinazoline D (3) caused 40 and 57% reductions in growth rate, respectively, at 0.2% incorporation in a pinto bean dietary assay28 against S. frugiperda (Table 3). Rotenone (Sigma-Aldrich, St Louis, MO, USA) was used as a positive control, and showed 75% reduction at 0.01% in the assay. Haenamindole (1) was reportedly non-cytotoxic towards Hep-3B, HeLa, K562 and HL60 cell lines,6 and was inactive in standard disk assays29, 30 conducted at 100 μg per disk against A. flavus (NRRL 6541), B acillus subtilis (ATCC 6051), Staphylococcus aureus (ATCC 29213) and Candida albicans (ATCC 90029).
MATERIALS AND METHODS
General experimental procedures
Melting points were measured using a Mel-Temp capillary melting point apparatus (Barnstead/Thermolyne, Dubuque, IA, USA), and are uncorrected. Optical rotations were acquired using a JASCO model DIP-1000 digital (Tokyo, Japan) or an AUTOPOL III automatic polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA). UV data were obtained in MeOH or EtOH with a Gilford Response or Varian Cary III UV/vis spectrophotometer. ECD data were recorded with an Olis Cary-17 spectrophotometer (1-cm cell). HPLC separations employed a Beckman System Gold instrument with a model 166P variable-wavelength UV detector connected to a 128 solvent module, equipped with a semi-preparative Apollo C18 column (Alltech, Associates, Deerfield, IL, USA 1.0 × 25 cm, 5 μm) under UV detection at 250 nm. 1H (300 or 600 MHz) and 13C (75 MHz) NMR data measurements were carried out using a Bruker AC-300, AMX-600 or AVANCE-600 spectrometer (Bruker Corp., Billerica, MA, USA). 2D NMR (COSY, HSQC, HMBC, NOESY) data were recorded on a Bruker AMX-600 or AVANCE-600 spectrometer. All NMR experiments were performed at room temperature, using CDCl3, acetone-d6 or DMSO-d6 as the solvent. Chemical shifts were referenced to residual solvent signals for CDCl3 (δH/δC, 7.26/77.2), acetone-d6 (δH/δC, 2.04/29.8) or DMSO-d6 (δH/δC, 2.50/39.5). HRESITOFMS data were collected on a Waters Q-ToF Premier mass spectrometer (Waters Corp., Milford, MA, USA). HRFABMS data were recorded on a VG ZAB-HF mass spectrometer (thioglycerol matrix; VG. Analytical, London, UK), while EIMS data were obtained at 70 eV using a VG Trio 1 quadrupole mass spectrometer (VG Analytical). Antiinsectan and antimicrobial assays were conducted using protocols that have been previously described.28, 29, 30
Fungal isolation, identification and fermentation
P. lanosum MYC-1813 was isolated from the mycelial surface of an unidentified wood decay fungus in a subalpine dry forest near milepost 43 of Highway 200 (Pu’u la’au, Hawaii, USA) using general procedures that have been previously described.31 A subculture of this isolate was deposited at the ARS USDA culture collection at the NCAUR under accession number NRRL 66231. Analysis of micromorphology, together with partial sequence analysis of the internal transcribed spacer region and D1 and D2 domains of the nuclear large subunit (28S) rDNA gene using ITS5 and NL4 as polymerase chain reaction and sequencing primers and a nucleotide-to-nucleotide BLAST query of the GenBank database using the internal transcribed spacer sequence returned a 100% sequence match (492 of 492) to P. lanosum and Penicillium kojigenum. P. lanosum, described in 1911, and P. kojigenum, described in 1961, are synonyms, and the first published name takes precedence. Sequence information was deposited in GenBank with the accession number KT698850. Compound 1 was also encountered in extracts of P. corylophilum (MYC-418=NRRL 28126), which was obtained from a sclerotium of A. flavus that had been buried in the Illinois River Valley Sand Field. A subculture of this isolate was also deposited at the NCAUR, and was assigned the accession number NRRL 28126. It was identified using an approach analogous to that described above. Briefly, a BLAST search of Genbank records using the internal transcribed spacer locus data from NRRL 28126 returned 99% similarity with P. corylophilum, P. obscurum and P. chloroleucon. P. corylophilum was described in 1901, while P. obscurum and P. chloroleucon were both described in 1923. A single base difference at a single locus is not sufficient to say that isolates are not conspecific—a much more detailed taxonomic study would be required in order to evaluate whether or not these species are actually identical. Because the three species are putative synonyms, the first published name P. corylophilum takes precedence, and was therefore assigned in this case. Sequence information for this culture was deposited in GenBank with the accession number KT698851.
Fermentation of P. lanosum MYC-1813 was carried out in two 500-ml Erlenmeyer flasks, each containing 50 g of rice. Distilled H2O (50 ml) was added to each flask, and the contents were soaked overnight before autoclaving at 15 lb in−12 for 30 min. The flasks were cooled to room temperature, inoculated with 3 ml of spore suspension and incubated for 30 days at 25 °C. After incubation, the fermented rice substrate was mechanically fragmented and then extracted repeatedly with EtOAc (3 × 100 ml per flask). The combined EtOAc extracts were filtered and evaporated to give 1.2 g of crude extract. A similar protocol was followed with P. corylophilum (MYC-418), although significantly less material was produced. Thus, the procedure used for isolation of metabolites from the P. lanosum MYC-1813 extract is presented below.
Extraction and isolation
The EtOAc extract of P. lanosum MYC-1813 was partitioned between n-hexane (8 ml × 3) and MeCN (8 ml). The resulting MeCN-soluble material (366 mg) was fractionated by silica gel column chromatography using n-hexane-EtOAc (160 ml of 100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80, 10:90 and 0:100) and EtOAc-MeOH (160 ml of 95:5 and 90:10) in a stepwise gradient, followed by pure MeOH (300 ml) to provide haenamindole (1; 32 mg; eluted as fraction 7 with 10:90 n-hexane-EtOAc) and 10 other fractions (Fr.1—Fr.6 and Fr.8—Fr.11). The most abundant additional fraction, Fr. 8 (21 mg out of 95 mg; eluted with 100% EtOAc), was subjected to reversed-phase HPLC (Alltech Apollo C18; 1.0 × 25 cm; flow rate, 2 ml min−1) with 33% MeCN in H2O over 60 min to afford an additional sample of 1 (3.7 mg; tR 44.5 min), as well as 2 (1.6 mg; tR 22.6 min), 3 (8.0 mg; tR 39.7 min), and sclerotigenin (9.7 mg; tR 14.3 min). Sclerotigenin was identified by comparison of its 1H NMR data to literature values.8
Haenamindole ( 1). Colorless plates (from MeOH containing a minimal amount of EtOAc); mp 186–189 °C; [α]D −59° (c 0.15, MeOH); UV (MeOH) λmax 248 (log ɛ 4.4), 281 (log ɛ 3.8); CD (33 μM, MeOH) λmax (Δɛ) 207 (−4.4), 218 (+12), 230 (+1.2), 254 (−11) and 285 (+1.2); 1H and 13C NMR data in acetone-d6, see Table 1; 1H NMR data in DMSO-d6 (Supplementary Figure S3) matched those reported for 1 in the same solvent6 (for direct comparison, see Supplementary Figure S2 in reference 6); key NOESY correlations (acetone-d6) H-10↔H-12, 20a; 9-OH↔H-20b, 21; EIMS (70 eV) m/z 478 (rel int 1), 385 (1), 293 (10), 275 (100), 233 (15), 145 (50), 131 (82), 91 (81), 77 (32); HRFABMS (PEG 600/thioglycerol/TFA) obsd m/z 513.2130 (M+H)+, calcd for C29H29N4O5, 513.2138.
2'-epi-Fumiquinazoline C ( 2). White solid; [α]D−108° (c 0.07, CHCl3); UV (EtOH) λmax 226 (log ɛ 4.2), 276 (log ɛ 3.6), 304 (log ɛ 2.6); CD (45 μM, EtOH) λmax (Δɛ) 212 (+20), 233 (−26), 254 (−6.5), 287 (−2.0), 307 (−3.1) and 317 (−2.5); 1H and 13C NMR data (acetone-d6), see Table 2; 1H NMR (CDCl3, 400 MHz) 8.33 (1H, d, J=7.7), 7.82 (1H, t, J=7.7 Hz), 7.75 (1H, d, J=7.7 Hz), 7.55 (1H, t, J=7.7 Hz), 7.51 (1H, d, J=7.6 Hz), 7.34 (1H, d, J=7.6 Hz), 7.33 (1H, t, J=7.6 Hz), 7.11 (1H, t, J=7.6 Hz), 6.87 (1H, br s), 5.78 (1H, m), 5.36 (1H, s), 3.92 (1H, q, J=7.0 Hz), 2.98 (1H, br d, J=15 Hz), 2.91 (1H, dd, J=15, 7.8 Hz), 2.08 (3H, s), 1.41 (3H, d, J=7.0 Hz); HMBC correlations (acetone-d6) H-7→C-9, 11; H-8→C-6, 10; H-9→C-7, 11; H-10→C-6, 8, 12; H-14→C-1, 4, 12, 15, 3'; H2-15→C-1, 14, 2', 3'; H3-16→C-3, 4; H-2'→C-15, 3'; H-5'→C-3', 7', 9'; H-6'→C-4', 8'; H-7'→C-5', 9'; H-8'→C-4', 6'; H-11'→C-2', 10'; H3-13'→C-10', 11'; key NOESY correlations (acetone-d6) H2-15↔H-2'; H-2'↔H2-15, H3-13'; H3-13'↔H-2'; HRESIMS m/z 444.1679 [M+H]+ (calcd for C24H22N5O4, 444.1672).
2'-epi-Fumiquinazoline D ( 3). White solid; [α]D−120° (c 0.34, CHCl3); UV (EtOH) λmax 227 (log ɛ 4.3), 270 (log ɛ 3.7), 306 (log ɛ 2.9), 317 (log ɛ 1.9); CD (38 μM, EtOH) λmax (Δɛ) 212 (+28), 233 (−26), 259 (−0.9), 307 (−8.0), and 319 (−6.2); 1H and 13C NMR data (CDCl3), see Table 2; HMBC correlations (CDCl3) H-7→C-9, 11; H-8→C-6, 10; H-9→C-7, 11; H-10→C-6, 8, 12; H-14→C-1, 4, 12, 15, 3'; H-15a→C-1, 14, 4'; H-15b→C-2', 3'; H3-16→C-3, 4; H-2'→C-15, 3', 10'; H-5'→C-3', 7', 9'; H-6'→C-4', 8'; H-7'→C-5', 9'; H-8'→C-4', 6'; H-11'→C-2', 10', 13'; H3-13'→C-10', 11'; key NOESY correlations (CDCl3) H-7↔H3-13'; H-15a↔H-2'; H-2'↔H-15a, H3-13'; H3-13'↔H-7, 2'; HRESIMS m/z 466.1484 [M+Na]+ (calcd for C24H21N5O4Na, 466.1491).
Methylation of haenamindole (1)
A solution of 1 (1.5 mg), TMSCHN2 (80 μl of 2 m solution in hexane) and MeOH (1 ml) was stirred at rt for 4 h until the initial yellow color of the solution disappeared and no starting material remained as detected by TLC. The solution was then evaporated and the reaction product was purified by RP HPLC (Alltech HS Hyperprep 100 BDS C18; 10 × 250 mm; flow rate, 2 ml min−1; 45–55% MeCN in H2O over 60 min) to afford the N-methoxy derivative of 1 (0.8 mg; tR 20 min; 52% yield). 1H NMR (CDCl3, 300 MHz) δ 8.09 (d, 8.1, H-4), 7.10–7.50 (m, 12H, H-5, 6, H-15—H-19, and H-29—H-33), 7.06 (d, 8.1, H-7), 6.15 (br s, C9-OH), 5.43 (br d, 8.4, H-21), 4.70 (br s, H-10), 4.35 (br s, H-12 and H-24), 3.76 (s, N-OCH3), 3.37 (br d, 13, H-27a), 2.97 (br d, 13, H-27b), 2.87 (dd, 17, 4.8, H-13a), 2.49 (dd, 17, 12, H-13b), 1.75 (br d, 15, H-20a), 1.34 (dd, 15, 8.1, H-20b); FABMS (3-NBA) obsd m/z 527 (M+H)+.
X-ray crystallographic analysis of haenamindole (1)
A thin, colorless plate (0.03 × 0.03 × 0.005 mm) was isolated from a sample crystallized from MeOH containing a minimal amount of EtOAc. Intensity data were collected at 150 K on a D8 goniostat equipped with a Bruker PHOTON100 CMOS detector at Beamline 11.3.1 at the Advanced Light Source (Lawrence Berkeley National Laboratory, Berkeley, CA, USA) using synchrotron radiation tuned to λ=1.2399 Å. Data collection frames were measured for a duration of 1 s at 0.5° intervals of ω with a maximum 2θ value of ~60°. The data frames were collected using the program APEX2 (v2014.5.0; Bruker Analytical X-ray Instruments, Inc., Madison, WI, USA) and processed using the SAINT program routine (v8.34A) within APEX2. The data were corrected for absorption and beam corrections based on the multi-scan technique as implemented in SADABS.32 The crystal structure contains two independent molecules, two water molecules at half occupancy and approximately 2.35 molecules of disordered MeOH of solvation. The non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms were included with the riding atom model (C-H, 1.00–0.95 Å; N–H, 0.88 Å; O–H, 0.84 Å). The N11 N–H bond was restrained to 0.90 Å, and the O9–H9 bond was restrained to 0.84 Å. H atoms on the disordered water molecules were not modeled. Attempts to model disordered solvent were unacceptable, so the contribution from the solvent was modeled with the SQUEEZE tool in the PLATON program.33 The calculated solvent contribution is equivalent to 9.38 MeOH molecules in the unit cell (2.35 per asymmetric unit). Crystallographic data for 1 have been deposited with the Cambridge Crystallographic Data Centre (deposition number CCDC 1417415). Copies of the data can be obtained, free of charge, on application to the Director, CCDC (12 Union Road, Cambridge CB2 1EZ, UK; e-mail: deposit@ccdc.cam.ac.uk.
Chiral TLC analysis of the acid hydrolyzate of haenamindole (1)
A sample of 1 (0.3 mg) was treated with 0.5 ml 6 n HCl and heated at 110 °C for 24 h. The resulting hydrolyzate, along with standards of d, l-Phe, l-Phe, d, l-Trp, l-Trp and d, l-β-Phe, were dissolved in n-BuOH/H2O/HOAc (4/1/1), and spotted on a chiral TLC plate (Macherey-Nagel, GmBH & Co., Duren, Germany). The same solvent system was used as the developing solvent. The Phe unit in the hydrolyzate co-chromatographed with the L-standard (Rf 0.67), and was well resolved from the d-isomer (Rf 0.54). The β-Phe in the hydrolyzate co-chromatographed with the d, l-standard (Rf 0.48). No Trp was detected in the acid hydrolyzate under these conditions.
Chiral HPLC analysis of the acid hydrolyzate of haenamindole (1)
The same acid hydrolysis procedure was applied to another sample of compound 1 (0.5 mg). The hydrolyzate obtained was dried, dissolved in H2O and subjected to HPLC analysis on a Chirex phase 3126 column (Phenomenex, Torrance, CA, USA; 4.6 × 50 mm; eluting with 95:5 2 mm aqueous CuSO4:MeCN at a flow rate of 0.5 ml min−1; detection at 254 nm). The retention times of the amino acids in the hydrolyzate were: β-Phe (9.30) and Phe (44.56). The retention times of authentic amino acid standards under the same conditions were d,l-β-Phe (9.27), d-Phe (49.46) and l-Phe (44.48). Based on these retention times, as well as co-injection, the absolute configuration of β-Phe could not be directly assigned, but the Phe in the hydrolyzate was confirmed to have the L-configuration.
References
Zhang, Y. et al. Novel antiinsectan oxalicine alkaloids from two undescribed fungicolous Penicillium spp. Org. Lett. 5, 773–776 (2003).
Li, C., Gloer, J. B., Wicklow, D. T. & Dowd, P. F. Thiersinines A and B: novel antiinsectan indole diterpenoids from a new fungicolous Penicillium species (NRRL 28147). Org. Lett. 4, 3095–3098 (2002).
Nelson, T. S., Beasley, J. N., Kirby, L. K., Johnson, Z. B. & Ballam, G. C. Isolation and identification of citrinin produced by Penicillium lanosum. Poult. Sci. 59, 2055–2059 (1980).
Beasley, J. N., Blalock, L. D., Nelson, T. S. & Templeton, G. E. The effect of feeding corn molded with Penicillium lanosum to broiler chicks. Poult. Sci. 59, 708–713 (1980).
Frisvad, J. C. et al. Four psychrotolerant species with high chemical diversity consistently producing cycloaspeptide A, Penicillium jamesonlandense sp. nov., Penicillium ribeum sp. nov., Penicillium soppi, and Penicillium lanosum. Int. J. Syst. Evol. Microbiol. 56, 1427–1437 (2006).
Kim, J. W. et al. Haenamindole, an unusual diketopiperazine derivative from a marine-derived Penicillium sp. KCB12F005. Bioorg. Med. Chem. Lett. 25, 5398–5401 (2015).
Ames, B. D. et al. Complexity generation in fungal peptidyl alkaloid biosynthesis: oxidation of fumiquinazoline A to the heptacyclic hemiaminal fumiquinazoline C by the flavoenzyme Af12070 from Aspergillus fumigatus. Biochemistry 50, 8756–8769 (2011).
Joshi, B. K., Gloer, J. B., Wicklow, D. T. & Dowd, P. F. Sclerotigenin: a new antiinsectan benzodiazepine from the sclerotia of Penicillium sclerotigenum. J. Nat. Prod. 62, 650–652 (1999).
Matsunaga, K., Shizuri, Y., Yamamura, S., Kawai, K. & Furukawa, H. Isolation and structure of citreoindole, a new metabolite of hybrid strain KO 0052 derived from Penicillium citreo-viride B. IFO 6200 and 4692. Tetrahedron Lett. 32, 6883–6884 (1991).
Hirsch, S., Miroz, A., McCarthy, P. & Kashman, Y. Etzionin, a new antifungal metabolite from a red sea tunicate. Tetrahedron Lett. 30, 4291–4294 (1989).
Barrow, C. J. et al. WIN 64821, a new competitive antagonist to substance P, isolated from an Aspergillus species: structure determination and solution conformation. J. Org. Chem 58, 6016–6021 (1993).
Maes, C. M., Potgieter, M. & Steyn, P. S. N.m.r. assignments, conformation, and absolute configuration of ditryptophenaline and model dioxopiperazines. J. Chem. Soc. Perkin Trans. 1, 861–866 (1986).
Kosemura, S., Ogawa, T. & Totsuka, K. Isolation and structure of asterin, a new halogenated cyclic penta-peptide from Aster tataricus. Tetrahedron Lett. 34, 1291–1294 (1993).
Morita, H., Nagashima, S., Takeya, K. & Itokawa, H. Astins A and B, antitumor cyclic pentapeptides from Aster tataricus. Chem. Pharm. Bull. 41, 992–993 (1993).
Morita, H., Nagashima, S., Takeya, K. & Itokawa, H. A novel cyclic pentapeptide with a β-hydroxy-γ-chloroproline from Aster tataricus. Chem. Lett. 23, 2009–2010 (1994).
Morita, H., Nagashima, S., Takeya, K. & Itokawa, H. Cyclic peptides from higher plants. Part 8. Three novel cyclic pentapeptides, astins F, G and H from Aster tataricus. Heterocycles 38, 2247–2252 (1994).
Corbin, J., Marsh, B. H. & Peters, G. A. N-γ-L-glutamyl-β-D-aminophenylpropanoic acid, a dipeptide from the aquatic fern, Azolla caroliniana. Phytochemistry 25, 527–528 (1986).
Needham, J., Kelly, M. T., Ishige, M. & Andersen, R. J. Andrimid and moiramides A-C, metabolites produced in culture by a marine isolate of the bacterium Pseudomonas fluorescens: structure elucidation and biosynthesis. J. Org. Chem 59, 2058–2063 (1994).
Fredenhagen, A. et al. Andrimid, a new peptide antibiotic produced by an intracellular bacterial symbiont isolated from a brown planthopper. J. Am. Chem. Soc. 109, 4409–4441 (1987).
Drandarov, K. Verbacine and verballocine, novel macrocyclic spermine alkaloids from Verbascum pseudonobile Stoj. et Stef. (Scrophulariaceae). Tetrahedron Lett. 36, 617–620 (1995).
Chi, Y.-M., Hashimoto, F., Yan, W.-M. & Nohara, T. Five novel macrocyclic spermine alkaloids from Incarvillea sinensis. Tetrahedron Lett. 38, 2713–2716 (1997).
Cole, R. J. & Cox, R. H. Handbook of Toxic Fungal Metabolites 708–712 (Academic Press, New York, USA, 1981).
Ghosh, A. C. & Ramgopal, M. Cyclic peptides from Penicillium islandicum. A review and a reevaluation of the structure of islanditoxin. J. Heterocyclic Chem. 17, 1809–1812 (1980).
Clardy, J., Springer, J. P., Buechi, G., Matsuo, K. & Wightman, R. Tryptoquivaline and tryptoquivalone, two tremorgenic metabolites of Aspergillus clavatus. J. Am. Chem. Soc. 97, 663–665 (1975).
Takahashi, C. et al. Fumiquinazolines A-G, novel metabolites of a fungus separated from a Pseudolabrus marine fish. J. Chem. Soc. Perkin Trans 1, 2345–2353 (1995).
Snider, B. B. & Zeng, H. Total syntheses of (−)-fumiquinazolines C, E, and H. Org. Lett. 4, 1087–1090 (2002).
Gao, X. et al. Fungal indole alkaloid biosynthesis: genetic and biochemical investigation of the tryptoquialanine pathway in Penicillium aethiopicum. J. Am. Chem. Soc. 133, 2729–2741 (2011).
Dowd, P. F., Berhow, M. A. & Johnson, E. T. Differential activity of multiple saponins against omnivorous insects with varying feeding preferences. J. Chem. Ecol. 37, 443–449 (2011).
Bauer, A. W., Kirby, W. M. M., Sherris, J. C. & Turck, M. Antibiotic susceptibility testing by a standard single disk method. Am. J. Clin. Pathol. 45, 493–496 (1966).
Wagenaar, M. M. & Clardy, J. Dicerandrols, new antibiotic and cytotoxic dimers produced by the fungus Phomopsis longicolla isolated from an endangered mint. J. Nat. Prod. 64, 1006–1009 (2001).
Höller, U., Gloer, J. B. & Wicklow, D. T. Biologically active polyketide metabolites from an undetermined fungicolous hyphomycete resembling Cladosporium. J. Nat. Prod. 65, 876–882 (2002).
Sheldrick, G. M. SADABS v2014/2 semi-empirical absorption and beam correction program. University of Göttingen, Germany.
Spek, A. L. PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. C 71, 9–18 (2015).
Acknowledgements
We are grateful to Professors Hyuncheol Oh of Wonkwang University and Jae-Hyuk Jang of the Korea Research Institute of Bioscience and Biotechnology (KRIBB) for providing a sample of haenamindole (1), which enabled direct comparison and verification that it was identical to our own sample of 1. We also thank Ms Nicole Krausert for technical support. Financial support was provided in part by a grant from the National Science Foundation (CHE-1011847). Crystallographic data were collected through the SCrALS (Service Crystallography at Advanced Light Source) program at Beamline 11.3.1 at the Advanced Light Source (ALS), Lawrence Berkeley National Laboratory. The ALS is supported by the US Department of Energy, Office of Energy Sciences Materials Sciences Division, under contract DE-AC02-05CH11231. Technical assistance from staff of the University of Iowa NMR and MS facilities is greatly appreciated. The USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This article is dedicated to the fond memory of the late Professor Lester Mitscher, a great scholar, teacher and Emeritus Editor of The Journal of Antibiotics.
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Hwang, I., Che, Y., Swenson, D. et al. Haenamindole and fumiquinazoline analogs from a fungicolous isolate of Penicillium lanosum. J Antibiot 69, 631–636 (2016). https://doi.org/10.1038/ja.2016.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2016.74
This article is cited by
-
Non-lipopeptide fungi-derived peptide antibiotics developed since 2000
Biotechnology Letters (2019)
-
A New L-alanine Derivative from the Mangrove Fungus Penicillium chrysogenum V11
Chemistry of Natural Compounds (2018)