Abstract
Growth inhibitors were isolated from an arctic strain of Trichoderma polysporum, and the structures were elucidated and the in vitro inhibitory effects of these compounds against Pythium iwayamai were investigated. Eleven compounds were isolated; four showed a concentration-dependent growth-inhibitory effect against P. iwayamai. None of these compounds have been reported previously as substances with antimicrobial activity against P. iwayamai. One of these four compounds inhibited the growth of the pathogen at 33 μg ml−1 concentration during a 15-day incubation at 20 °C. This effect was comparable to that of chloroneb (1: 1,4-dichloro-2,5-dimethoxybenzene), a fungicide with activity against P. iwayamai. Thus, the results of the present study show that the arctic strain of T. polysporum can be an effective source of antibiotics with activity against the snow rot pathogen, P. iwayamai.
Similar content being viewed by others
Introduction
Trichoderma spp. are common inhabitants of roots, soil and foliar environments; these organisms represent the most frequently isolated soil fungi.1 Their rapid growth and ability to metabolize a wide variety of substrates have enabled Trichoderma spp. to become the predominant components of the soil mycobiota in such diverse ecosystems as agricultural fields, pastures, grassland, forests, salt wetlands, deserts and polar regions.2, 3 T. polysporum (Link ex. Pers.) Rifai is a psychrotrophic species of the genus and is distributed worldwide throughout temperate to arctic regions.4, 5 Pythium snow rot is one of the most destructive diseases of wheat and grasses in Japan,6 Canada7 and the United States.8, 9 Pythium iwayamai Ito is a major causal agent of the disease.8 However, only a limited number of chemicals are effective against Pythium snow rot.10, 11 For these reasons, novel compounds are needed for disease control. In our previous study, we isolated T. polysporum strains from a moss, Sanionia uncinata (Hedw.) Loeske, growing in the high arctic wetlands on Spitsbergen Island, Svalbard, Norway.12 The arctic T. polysporum strains grew and infected the moss without any symptoms at 0 °C.12 Given that T. polysporum from temperate regions has been reported to produce some antibiotics,13, 14, 15 we thought that arctic isolates of T. polysporum also might produce antibiotics against pathogens known to grow in a low-temperature environment. We conducted confrontation experiments between an arctic T. polysporum and several snow molds and found that the arctic T. polysporum showed a growth-inhibitory effect against P. iwayamai. (Figures 1a and b)
(a) Co-cultures of Trichoderma polysporum OPU1571 (Tp) and Pythium iwayamai OPU1452 (Pi) on potato dextrose agar. P. iwayamai hyphae were inhibited before coming into contact with T. polysporum hyphae. (b) A magnified view. A full color version of this figure is available as Supplementary Figure S1.
Herein we describe the production of growth inhibitors from this arctic strain of T. polysporum, and the isolation, structure determination and growth-inhibitory effects on P. iwayamai of these inhibitors.
Results and Discussion
In this study, we used T. polysporum OPU1571, an arctic strain. The growth of OPU1571 was very slow at low temperatures, necessitating overly long incubation times. We previously reported that arctic T. polysporum strains exhibited growth at temperature ranges of 0–25 °C or 0–28 °C, with maximum growth observed at ~20 °C.12 Therefore, we cultured our arctic strain of T. polysporum at each of two incubation conditions (at 4 °C for 1 year, and at 20 °C for 53 days), and confirmed that the HPLC chromatograms of the resulting fungal extracts were very similar (Supplementary Figure S2). On the basis of this result, T. polysporum OPU1571 was cultured at 20 °C for 53 days and compounds 2–12 were isolated from the resulting fungal extract (Figure 2). Details of the isolation and purification of these compounds are provided in the ‘Experimental procedure’ section and in Supplementary Figure S3.
Structure elucidation of 3
The molecular formula of 3 was established as C9H12O3 by ESI-TOF-MS; this compound has 4 degrees of unsaturation. The 1H-NMR spectrum of 3 showed three sets of methylene proton signals, a methoxy proton signal (δ 3.73), a methine proton signal (δ 4.57) adjacent to an oxygen atom, and two olefin proton signals (δ 6.06 and δ 7.08 (J=15.5 Hz each); Supplementary Figure S4). The olefin group of 3 was determined to be in the trans configuration by the spin coupling constants (δ 6.06 and δ 7.08 (J=15.5 Hz)). The 13C NMR and HSQC spectra of 3 assigned a carbonyl carbon (δ 167.6) and a methoxy carbon (δ 51.7), two sp2 carbons (δ 117.7 and δ 154.4), three sp3 methylene carbons (δ 34.0, δ 38.4 and δ 49.9), a sp3 methine carbon (δ 72.8), and a fully substituted carbon (δ 80.8) adjacent to oxygen atom (Supplementary Figure S5 and S6). The presence of a methyl acrylate moiety in 3 was deduced from HMBC correlations from 9-H3 to C-1, and from 2-H to C-1 and C-3. The HMBC from 5-H to C-4 and C-8, from 6-H to C-4 and C-8, and from 7-H to C-5, C-6 and C-8 showed the presence of an epoxycyclopentane moiety in 3.
Furthermore, we observed HMBC correlations from 2-H (δ 6.06) and 3-H (δ 7.08) to the fully substituted carbon (δ 80.8) adjacent to the oxygen atom at the C-4 position of an epoxycyclopentane moiety in 3 (Figure 3 and Supplementary Figure S7). These results indicated that the C-3 position of the methyl acrylate moiety was connected to the C-4 positions of the epoxycyclopentane moiety; hence the structure of 3 was established as (E)-methyl 3-(6-oxabicyclo[3.1.0]hexan-1-yl)prop-2-enoate, as shown in Figure 3. Unfortunately, we could not determine the stereochemistry at C-4 and C-5 in this study.
Growth-inhibitory effect of 2–7, 9 and 10 against P. iwayamai
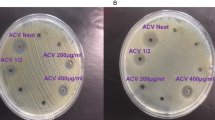
The growth-inhibitory effect against P. iwayamai was tested for 2–7, 9 and 10. Compounds 8, 11 and 12 were not tested because the amounts of these compounds were limited. Chloroneb (1), a known antifungal agent, was used as a positive control. Compounds 3, 6, 7 and 10 showed a concentration-dependent growth-inhibitory effect on P. iwayamai at 5 days. In particular, treatment with 7 (at 33 μg ml−1 concentration) significantly inhibited the growth of P. iwayamai. Growth-inhibitory effects were not observed with 2, 4, 5 or 9 at 33 μg ml−1 concentration (Figure 4a).
Growth-inhibitory activity of 1–7, 9 and 10 on Pythium iwayamai. Growth-inhibitory activities on P. iwayamai were measured after culturing for 5 days (a), 7 days (b) or 15 days (c). Statistical significance of data is given by the P-value (*P<0.05; **P<0.01). Growth-inhibitory activity of 2–7, 9 and 10 for P. iwayamai. A full color version of this figure is available as Supplementary Figure S8.
To confirm sustained growth-inhibitory activity against P. iwayamai, 3, 6, 7 and 10 were tested for growth inhibition during extended incubation intervals. After 7 days, the growth-inhibitory effects of 6 and 10 were no longer seen, and the effect of 3 was weakened, whereas 7 (at 33 μg ml−1 concentration) continued to exhibit a significant growth-inhibitory effect (Figure 4b). Furthermore, 7 (at 33 μg ml−1 concentration) showed a significant growth-inhibitory effect even after 15 days of exposure (Figure 4c).
Thus, in this study, we isolated multiple compounds from extracts of an arctic strain of T. polysporum OPU1571; several of the resulting substances (including 3, 6, 7 and 10) inhibited the growth of the snow rot pathogen P. iwayamai. The structure of 3 was determined by detailed analysis of the NMR and MS spectra. Compound 3 has a five-membered ring with an epoxide in its structure. Other fungally derived antimicrobials having five-membered rings with epoxides include antibiotic 2188,16 dermadin,17, 18, 19 trichoviridin,18, 20 isonitroin21 and related compounds.22, 23, 24 Among these antimicrobials, those having isonitrile groups showed antibacterial and antifungal activities. However, the growth-inhibitory activities of these compounds against the snow rot organism (P. iwayamai) were not reported. Therefore, compound 3 is the first example (to our knowledge) of an anti-Pythium snow rot agent having a five-membered ring with an epoxide moiety. Chloroneb (1) is the only fungicide approved for use against P. iwayamai,11 although cyazofamid and amisulbrom recently have been permitted for use against the pathogen.12 Chloroneb (1) is an aromatic hydrocarbon fungicide; the compound causes lysis of the inner mitochondrial membrane in sensitive microorganisms, with further attack on nuclear and other membranes at high doses.25 The mechanism(s) of action of 3, 6, 7 and 10 are not known; we intend to study these in future work.
Experimental procedure
General procedures
Melting points were measured on a Yanagimoto micro-melting point apparatus (Yanaco New Science, Inc., Kyoto, Japan). Optical rotations were measured on a JASCO DIP-1000 (JASCO Co., Ltd., Tokyo, Japan). CD spectra were obtained with a JASCO J-820 spectrophotometer (JASCO). UV spectra were recorded on an Amersham Biosciences Ultrospec 2100 spectropolarimeter (GE Healthcare Japan Corp., Tokyo, Japan). Measurements of IR spectra were performed with a JASCO FT/IR-4100 spectrometer on a KBr cell (JASCO). NMR spectra (both 1D and 2D) were obtained on a Bruker AVANCE-400 (400.13 MHz for 1H and 100.61 MHz for 13C) spectrometer (Bruker Bio Spin K. K., Kanagawa, Japan). Multiplicity of signals is abbreviated as follows: s=singlet, d=doublet, dd=doublet of doublets, dt=doublet of triplets, t=triplet, q=quartet, m=multiplet and br=broad. EI and CI-MS spectra were obtained from a JEOL JMS-600W spectrometer (JEOL Ltd., Tokyo, Japan). Electrospray ionization ESI-TOF-MS spectra were obtained from a JEOL T100LP spectrometer (JEOL).
The isolation of metabolites was performed by medium-pressure liquid chromatography on a silica gel column (25 × 250 mm, YAMAZEN, Osaka, Japan) and preparative HPLC using a Senshu SSC-3160 pump (Senshu Scientific Co., Ltd., Tokyo, Japan). Preparative HPLC was performed using a LC-20AT prominence pump (flow rate 2 ml min−1, Shimadzu Corp., Kyoto, Japan) and an Inertsil ODS-P column (10 × 250 mm, GL science Inc., Tokyo, Japan) in a CO-965 column oven (temperature at 30 °C, JASCO), and monitored with a SPD-20A UV detector (Shimadzu). A BioShaker PERSONAL-11 (TAITEC Co., Ltd., Saitama, Japan) was used for the incubation of P. iwayamai as a test microbe. The OD of the microbial culture was measured at a wavelength of 600 nm using a Bactomonitor BACT-500 instrument (Intertech, Inc., Tokyo, Japan).
Chemicals
Chloroneb (1) was used as a positive control and was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The biological assay was performed in peptone-yeast-glucose broth, which was formulated using (per liter water) 1.0 g polypeptone (Nihon Pharmaceutical Co., Ltd, Tokyo, Japan), 1.0 g yeast extract (Oriental Yeast Co., Ltd., Tokyo, Japan), and 10.0 g d-glucose (Wako Pure Chemical Industries, Ltd.).
Statistical analysis
Data values are presented as mean±s.d. from three independent experiments. Differences among experimental groups were compared with non-repeated measures analysis of variance with post hoc Dunnett’s test. A value of P<0.05 was considered statistically significant.
Organisms
T. polysporum strain OPU1571 was isolated from brown discolored S. uncinata from the land area of wetlands in Longyearbyen (78° 15′N, 15° 30′E), Spitsbergen, Svalbard archipelago, Norway, in August 2002, using water agar composed of 1.5% agar (Wako).12 P. iwayamai strain OPU1452 was isolated from Kentucky bluegrass turf in a golf course fairway in mid-eastern Hokkaido, Japan (43° 04′N, 141° 21′E). Both species were identified based on DNA sequence analysis of the internal transcribed spacers (ITS) region and morphological observations.12, 26
Growth-inhibitory effect on P. iwayamai by living T. polysporum
T. polysporum OPU1571 and P. iwayamai OPU1452 were placed on potato dextrose agar (Sigma Aldrich, St Louis, MO, USA) in the same Petri dish ~7 cm apart; the plate was incubated for 26 days at 0 °C. P. iwayamai hyphal growth was inhibited before hyphae came into contact with those of T. polysporum (Figures 1a and b). The experiment was repeated three times by using a single plate per repetition. All the experiments showed the same response between T. polysporum and P. iwayamai.
Culture, extraction and isolation
The culture medium consisted of moist rice, autoclaved at 120 °C for 20 min before inoculation. T. polysporum OPU1571 was inoculated into each of 25 Roux flasks containing 150 g of moist rice per flask and cultured at 20 °C for 53 days. The fermented rice was extracted with MeOH and the organic layer was evaporated. The resulting extract was suspended in H2O and extracted with ethyl acetate (EtOAc) before the organic layer was evaporated in vacuo. The EtOAc extract (12.8 g) was resuspended successively in individual organic solvents (hexane, benzene, CHCl3 and MeOH); at each step the solvent-soluble layer was recovered and evaporated in vacuo. Purification was performed using an antimicrobial test and HPLC analysis-guided fractionation. The hexane extract (6.1 g) was chromatographed using a silica gel column (40 × 200 mm) with a solvent system of CHCl3 (1000 ml), CHCl3-MeOH (400 ml at 100:1; 250 ml at 50:1; 250 ml at 20:1) acetone (250 ml), EtOH (250 ml) and MeOH (500 ml), to yield seven fractions.
The first fraction was purified by medium-pressure liquid chromatography on a silica gel column (25 × 250 mm, YAMAZEN); eluted with benzene–acetone (100:1) yielded (E)-6-(pent-1-enyl)-2H-pyran-2-one (2: 71 mg).27, 28
The second fraction was suspended in MeOH and filtered. The residual solid was confirmed to contain ergosterol peroxide (128 mg). The third fraction was purified by HPLC on a silica gel column (10 × 250 mm, GL science, Tokyo, Japan) using benzene-MeOH (15:1) followed by benzene–acetone (5:1); cyclonerodiol (4: 183 mg)29, 30 was obtained from this purification. The fourth fraction was purified by HPLC on a silica gel column using benzene–acetone (20:1) followed by CHCl3-MeOH (30:1); mevalonolactone (9: 271 mg) was obtained from this purification. The fifth fraction was purified by HPLC on a silica gel column using CHCl3-MeOH (30:1) and (10:1); mevalonic acid (17 mg) and anhydromevalonolactone (8: 3 mg)31 were obtained from this purification.
The benzene extract (1.7 g) was purified by medium-pressure liquid chromatography on a silica gel column using CHCl3-acetone (20:1) followed by HPLC on a silica gel column using benzene-MeOH (10:1) and CHCl3–acetone (3:1); a dihydroosmundalactone (7: 18 mg)32, 33 was obtained from this purification.
The CHCl3 extract (4.3 g) was suspended in CH3CN and filtered. The filtrate (2.6 g) was purified by medium-pressure liquid chromatography on a silica gel column using benzene–acetone (5:1) followed by further purification by HPLC on a silica gel column. Elution with hexane–acetone (5:1) yielded methyl p-hydroxyphenylacetate (11: 6 mg);34 a subsequent elution with benzene–acetone (10:1) yielded harzialactone A (5: 49 mg)35, 36, 37 and latifolicinin C (12: 2 mg);38 a subsequent elution with CHCl3-MeOH (50:1) yielded 2-(4-hydroxyphenyl)ethanol (10: 10 mg);39 a subsequent elution with benzene–acetone (2:1) followed by hexane–acetone (1:1) yielded 2-anhydromevalonic acid (6: 11 mg)40 and 4-hydroxybutyric acid (4 mg); and a subsequent elution with CHCl3-MeOH (50:1) followed by (20:1) yielded (E)-methyl 3-(6-oxabicyclo[3.1.0]hexan-1-yl)prop-2-enoate (3: 17 mg). The structures of the isolated compounds 2–12 are shown in Figure 2.
Compound 3
Colorless oil: [α]20D −3.7°(c 1.0, MeOH); HR-ESI-TOFMS m/z: 169.0884 [(M+H)+, 169.0864 calculated for C9H13O3]. 1H-NMR (CDCl3) δ 1.68 (1H, m, 6-H), 1.73 (1H, m, 7-H), 1.80 (1H, dd, J=4.7, 14.7 Hz, 5-H), 2.06 (1H, m, 6-H), 2.10 (1H, m, 5-H), 2.22 (1H, m, 7-H), 3.73 (3-H, s, 9-H), 4.57 (1H, s, 8-H), 6.06 (1H, d, J=15.5 Hz, 2-H), 7.08 (1H, d, J=15.5 Hz, 1H); 13C NMR (CDCl3) δ 34.0 (C-7), 38.4 (C-6), 49.9 (C-5), 51.7 (C-9), 72.8 (C-8), 80.8 (C-4), 117.7 (C-2), 154.4 (C-3) and 167.6 (C-1).
Growth-inhibitory activity against P. iwayamai
This assay was performed using pathogenic snow rot, P. iwayamai OPU1452. The strain was incubated in peptone-yeast-glucose broth with the addition of indicated concentrations (1.7, 3.3 and 33 μg ml−1) of the test compounds; cultures then were incubated at 20 °C for 15 days using a BioShaker. Growth of P. iwayamai was measured each day by the OD600 of the broth culture using a Bactomonitor. Chloroneb (1.7, 3.3 and 33 μg ml−1) was used as a positive control in this experiment.
References
Harman, G. E., Howell, C. R. & Chet, I. Trichoderma species-opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2, 43–56 (2004).
Klein, D., Eveleigh, E. in Ecology of Trichoderma. Trichoderma and Gliocladium. Basic Biology, Taxonomy and Genetics (eds Kubicek, C. P. & Harman, G. E. 57–73 Taylor & Francis, London, (1998).
Elvebakk, A., Gjærum, H. B., Sivertsen, S. in Part 4. Fungi II. Myxomycota, Oomycota, Chytridiomycota, Zygomycota, Ascomycota, Deutromycota, Basidiomycota: Uredinales and Ustilaginales. A Catalogue of Svalbard Plants, Fungi, Algae and Cyanobacteria (eds Elvebakk, A. & Prestrud, P. 207–259 Norsk Polarinstitutt, Oslo, (1996).
Goldfarb, B., Nelson, E. E. & Hansen, E. M. Trichoderma spp.: growth rates and antagonism to Phellinus weirii in vitro. Mycologia 81, 375–381 (1989).
Zabawski, J. Soil microfungi of peats in the Hornsund region (West Spitsbergen). Acta Univ. Wratislaviensis 525, 269–279 (1982).
Takamatsu, S. Ecological study of Pythium snow rot of wheat and barley. Spec. Bull. Fukui Agric. Exp. Stn. 9, 1–135 (1989).
McBeath, J. H. Snow mold: winter turfgrass nemesis. Golf Course Manag. 71, 121–124 (2003).
Lipps, P. E. & Bruehl, G. W. Snow rot of winter wheat in Washington. Phytopathology 68, 1120–1127 (1978).
Abad, Z. G., Shew, H. D. & Lucas, L. T. Characterization and pathogenicity of Pythium species isolated from turf grass with symptoms of root and crown rot in North Carolina. Phytopathology 84, 913–921 (1994).
Food and Agricultural Materials Inspection Center (FAMIC) (2015) Available at: http://www.famic.go.jp/. Accessed on 30 October 2015.
Green and Safety Promoters Association Green Noyaku Souran 2007 246 Green and Safety Promoters Association Ltd., Tokyo, (2007).
Yamazaki, Y. et al. Characterization of Trichoderma polysporum from Spitsbergen, Svalbard archipelago, Norway, with species identity, pathogenicity to moss, and polygalacturonase activity. Fungal Ecol. 4, 15–21 (2011).
Iida, A., Yoshimatsu, S., Sanekata, M. & Fujita, T. Fungal metabolites. IV. Synthesis of an antibiotic peptide, trichosporin B-V, from Trichoderma polysporum. Chem. Pharm. Bull. 38, 2997–3003 (1990).
Jassim, H. K., Foster, H. A. & Fairhurst, C. P. Biological control of Dutch elm disease: larvicidal activity of Trichoderma harzianum, T. polysporum and Scytalidium lignicola in Scolytus scolytus and S. multistriatus reared in artificial culture. Ann. Appl. Biol. 117, 187–196 (1990).
Iwatsuki, M. et al. Antitrypanosomal peptaibiotics, trichosporins B-VIIa and B-VIIb, produced by Trichoderma polysporum FKI-4452. J. Antibiot. 63, 331–333 (2010).
Ohsugi, K., Ichida, J. & Takahashi, E. (Kureha Chemical Ind. Co., Ltd) Antibiotic compound. DE3326518 A1 19840126 (1984).
Coats, J. H., Meyer, C. E. & Pyke, T. R. (The Upjohn Company) Antibiotic dermadin. US3627882A (1971).
Tamura, A., Kotani, H. & Naruto, S. Trichoviridin and dermadin from Trichoderma sp. TK-1. J. Antibiot. 28, 161–162 (1975).
Brewer, D. et al. Isonitrile acids from cultures of the fungus Trichoderma hamatum (Bon.) Bain. aggr., X-ray structure. J. Chem. Soc. Chem. Commun. 23, 1061–1062 (1979).
Nobuhara, M. et al. A fungal metabolite, novel isocyano epoxide. Chem. Pharm. Bull. 24, 832–834 (1976).
Fujiwara, A. et al. Isonitrile antibiotics, a new class of antibiotics with an isonitrile group. I. Fermentation, isolation and characterization of isonitrile antibiotics. Agric. Biol. Chem. 46, 1803–1809 (1982).
Baldwin, J. E. et al. The total synthesis of (±)- isonitrin B (deoxytrichoviridin). Synlett 1, 9–14 (1989).
Baldwin, J. E., O’Neil, I. A. & Russell, A. T. Isonitrin A: revision of the structure and total synthesis in racemic form. Synlett 8, 551–552 (1991).
Baldwin, J. E., Adlington, R. M., O’Neil, I. A., Russell, A. T. & Smith, M. L. The total synthesis of (±)-trichoviridin. Chem. Commun. 1, 41–42 (1996).
Lyr, H. & Werner, P. On the mechanism of action of the fungicide chloroneb. Pest. Biochem. Physiol. 18, 69–76 (1982).
Masumoto, S., Shigyo, T. & Tojo, M. Pythium snow blight of Kentucky bluegrass turf in a golf course in Hokkaido, Japan. J. Jpn. Soc. Turfgrass Sci. 38, 33–36 (2009).
Claydon, N., Allan, M., Hanson, J. R. & Avent, A. G. Antifungal alkyl pyrones of Trichoderma harzianum. Trans. Br. Mycol. Soc. 88, 503–513 (1987).
Parker, S. R., Cutler, H. G., Jacyno, J. M. & Hill, R. A. Biological activity of 6-Pentyl-2H-pyran-2-one and its analogs. J. Agric. Food Chem. 45, 2774–2776 (1997).
Nozoe, S., Goi, M. & Morisaki, N. Structure of cyclonerodiol. Tetrahedron Lett. 15, 1293–1296 (1970).
Cutler, H. G. et al. Cyclonerodiol from a novel source, Trichoderma koningii: Plant growth regulatory activity. Agric. Biol. Chem. 55, 243–244 (1991).
Frauendorfer, F. & Schieberle, P. Changes in key aroma compounds of Criollo cocoa beans during roasting. J. Agric. Food Chem. 56, 10244–10251 (2008).
Hollenbeak, K. H. & Kuehne, M. E. The isolation and structure determination of the fern glycoside osmundalin and the synthesis of its aglycone osmundalactone. Tetrahedron 30, 2307–2316 (1974).
Zhu, L., Talukdar, A., Zhang, G., Kedenburg, J. P. & Wang, P. G. A divergent synthesis of uncommon sugars from furanaldehyde. Synlett 10, 1547–1550 (2005).
Fragoso-Serrano, M. et al. Profiling of alkaloids and eremophilanes in miracle tea (Packera candidissima and P. bellidifolia products. J. Nat. Prod. 75, 890–895 (2012).
Chen, B., Yin, H.-F., Wang, Z.-S. & Xu, J.-H. New synthesis of harzialactone A via kinetic resolution using recombinant Fusarium proliferatum lactonase. Tetrahedron Asymmetry 21, 237–240 (2010).
Kotkar, S. P., Suryavanshi, G. S. & Sudalai, A. A short synthesis of (+)-harzialactone A and (R)-(+)-4-hexanolide via proline-catalyzed sequential α-aminooxylation and Horner–Wadsworth–Emmons olefination of aldehydes. Tetrahedron Asymmetry 18, 1795–1798 (2007).
Amagata, T., Usami, Y., Minoura, K., Ito, T. & Numata, A. Cytotoxic substances produced by a fungal strain from a sponge: physico-chemical properties and structures. J. Antibiot. 51, 33–40 (1998).
Begum, S. et al. Chemical constituents of Cordia latifolia and their nematicidal activity. Chem. Biodivers. 8, 850–861 (2011).
Park, C. H. et al. Phenolic constituents of Acorus gramineus. Arch. Pharm. Res. 34, 1289–1296 (2011).
Xu, J., Aly, A. H., Wray, V. & Proksch, P. Polyketide derivatives of endophytic fungus Pestalotiopsis sp. isolated from the Chinese mangrove plant Rhizophora mucronata. Tetrahedron Lett. 52, 21–25 (2011).
Acknowledgements
Part of this study was supported by a grant from the Japan Society for the Promotion of Science (grant-in-aid for scientific research no. 15K00626) to MT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Kamo, M., Tojo, M., Yamazaki, Y. et al. Isolation of growth inhibitors of the snow rot pathogen Pythium iwayamai from an arctic strain of Trichoderma polysporum. J Antibiot 69, 451–455 (2016). https://doi.org/10.1038/ja.2015.130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2015.130