Abstract
Two new polyketides, 6,8,5′6′-tetrahydroxy-3′-methylflavone (1) and paecilin C (2), together with six known analogs secalonic acid D (3), secalonic acid B (4) penicillixanthone A (5), emodin (6), citreorosein (7) and isorhodoptilometrin (8) were obtained from a broth of gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023. Compounds 1 and 6–8 had significant antifouling activity against Balanus amphitrite larvae settlement with EC50 values of 6.7, 6.1, 17.9 and 13.7 μg ml−1, respectively, and 3–5 showed medium antibacterial activity against four tested bacterial strains. This was the first report of antibacterial activity of 3–5 against marine bacteria and antifouling activity of 6–8 against marine biofouling organism’s larvae. The results indicated that gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023 strain could produce antifouling and antibacterial compounds that might aid the host gorgonian coral in protection against marine pathogen bacteria, biofouling organisms and other intruders.
Similar content being viewed by others
Introduction
Gorgonian corals appear to have few predators and survive in the highly competitive and hostile marine environment, mainly relying on a series of secondary metabolites accumulating in their bodies or releasing to their surroundings, which have important roles in protecting the colonies against grazing, feeding and marine biofouling organisms.1 A variety of studies suggested that coral-associated microbe was a possible first line of chemical defense for corals, possibly through producing bioactive substances.2, 3 Recent studies showed that gorgonian corals had a large and diverse fungal community, and Penicillium was one of the most diverse and common genus in gorgonian corals.4, 5 However, few attentions were paid to the secondary metabolites of gorgonian coral-associated fungi and their possible chemical defense roles for their hosts.
In our preliminary experiment, we found that the ethyl acetate extract of a culture broth of the marine-derived fungus Penicillium sp. SCSGAF 0023 isolated from South China Sea gorgonian coral Dichotella gemmacea exhibited significant antibacterial activity against Escherichia coli and Bacillus subtilis and antifouling activity against Balanus amphitrite larvae settlement. Further investigation on the chemical constituents of the extract led to the isolation of eight polyketides including two new compounds. For these compounds, antifouling and antibacterial activities were evaluated. In this paper, we describe the isolation, structure elucidation and bioactivities of these secondary metabolites from the marine gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023 strain.
Results
Isolation and identification of compounds
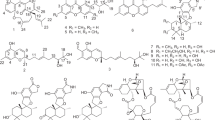
Totally, eight polyketides were obtained from the ethyl acetate extract of the broth of Penicillium sp. SCSGAF 0023 strain. The structures of these compounds were identified by spectroscopic analysis and comparison with published data. They were 6,8,5′6′-tetrahydroxy-3′-methylflavone (1), paecilin C (2), secalonic acid D (3),6 secalonic acid B (4),7 penicillixanthone A (5),8, 9 emodin (6),10 citreorosein (7)10 and isorhodoptilometrin (8)11 (Figure 1). Among them, 1 and 2 were new compounds, and 3 was the main secondary metabolite of the strain accounting about 2.5% of the crude extract.
Compound 1 was obtained as white power, having the molecular formula C16H12O6 as deduced from NMR spectra and (+)-ESIMS. The 1H NMR spectrum showed the signals of one methyl at δH 2.22 (3H, s), five aromatic protons at δH 6.35 (1H, d, J=1.5 Hz), 6.28 (1H, d, J=1.5 Hz), 6.26 (1H, d, J=1.5 Hz), 6.24 (1H, s) and 6.23 (1H, d, J=1.5 Hz). The 13C and DEPT NMR spectra exhibited 16 carbons including 1 methyl (δC 20.4), 5 methines (δC 95.0, 100.2, 101.3, 110.2 and 112.9) and 10 quaternary carbons (δC 105.4, 113.2, 140.8, 158.4, 160.3, 161.4, 163.3, 166.2, 166.3 and 184.0). These data suggested that 1 should have a flavonoid skeleton.12, 13, 14, 15 This suggestion was proved by the heteronuclear multiple bond connectivity (HMBC) spectrum (Figure 2). In the HMBC spectrum, HMBC correlations from H-5 (δH 6.35) to C-4 (δC 184.0)/C-4a (δC 105.4)/C-6 (δC 166.2)/C-7 (δC 100.2)/C-8a (δC 160.3), from H-7 to C-5(95.0)/C-6/C-8 (δC 163.3), suggested the oxygenation of C-6 and C-8. HMBC correlations from H-3 (δH 6.24) to C-4/C-4a (δC 105.4)/C-2 (δC 166.3)/C-1′ (δC 113.2) indicated that 1 was a flavone. Furthermore, HMBC correlations from H-7′ (δH 2.22) to C-2′ (δC 110.2)/C-3′ (δC 140.8)/C-4′ (δC 112.9), from H-2′ (δH 6.28) to C-1′/C-4′/C-6′ (δC 161.4), from H-4′ (δH 6.26) to C-5′ (δC 158.4)/C-6′, suggested the oxygenation of C-5′/C-6′ and one methyl attached on C-3′. Based on the above data, the structure of 1 was determined as shown in Figure 1.
Compound 2 was obtained as a pale yellow gum, having a molecular formula of C32H30O14 as deduced from NMR spectra and (+)-ESIMS. The 1H NMR spectrum showed the signals of two phenolic hydroxyl protons at δ 11.92 (1H, s), 12.03 (1H, s), two sets of doublets of AB-aromatic system protons at δ 6.64 (1H, d, J=8.0 Hz), 7.52 (1H, d, J=8.5 Hz), 6.63 (1H, d, J=8.0 Hz), 7.54 (1H, d, J=8.5 Hz), two oxymethines at δ 4.82 (1H, d, J=6.5 Hz), 4.10 (1H, s), two methoxyl groups at δ 3.79 (3H, s), 3.77 (3H, s) and two methyls at δ 1.36 (3H, d, J=7.0 Hz), 1.11 (3H, d, J=6.5 Hz). The 13C and DEPT NMR spectra exhibited 32 carbons including 2 methyls, 2 methoxyls, 4 methylenes, 2 methines, 2 oxymethines, 2 oxyquaternary carbons, 12 aromatic carbons, 4 carboxyl groups and 2 ketone groups (δC 195.9, 194.1). The signals of all carbon resonances appeared in pairs, which suggested that 2 should be a dimmer. The 1H and 13C NMR data of 2 showed great similarity to those of paecilin A16 with the only obvious differences of the chemical shifts of H-9′ (from δH 4.65 (d, J=7.5 Hz) to δH 4.10 (br s)), H-10′ (from δH 2.87 (m) to δH 2.39 (m)), H-11′ (from δH 2.30 (dd, J=8.5, 17 Hz), 1.96 (dd, J=11, 17 Hz) to δH 2.67 (dd, J=8.0, 17 Hz), 2.46 (m)), C-9′ (from δC 82.3 to δC 76.2), C-10′ (from δC 33.8 to δC 30.7) and C-11′ (from δC 35.2 to δC 40.2), which indicated that 2 might be an epimer of paecilin A. The 2D NMR spectra including HSQC, HMBC and 1H–1H COSY spectra (Figure 2) of 2 proved that 2 had the same planar structure as paecilin A. The relative configuration of 2 was further inferred from the NOESY spectrum (Figure 3) and coupling constants of JH-9/H-10 and JH-9′/H-10′. The large coupling constant of JH-9/H-10 (6.5 Hz) and comparison of NMR data of H-9/H-10/H-11, C-9/C-10/C-11 in 2 and paecilin A, combined no NOE correlation between H-9 and Me-13 observed in the NOESY spectrum, suggested the relative configurations of H-9β, H-10β and Me-13α in 2 that were the same as those in paecilin A. In addition, according to the Karplus curve, the small coupling constant of JH-9′/H-10′ (H-9′ appeared as singlet) indicated a dihedral angle of ∼90 ° between H-9′ and H-10′. The presence of NOE correlation between H-9' and Me-13' indicated that H-9′ and Me-13′ were cis- configuration, based on the dihedral angle measurement and small coupling constant of JH-9′/H-10′, which suggested the relative configurations of H-9′α, H-10′β and Me-13′α in 2. Based on the above data, the structure of 2 was inferred as shown in Figure 1. The specific optical rotation of 2 (−71.35o) was opposite to that of paecilin A (+61.60o).
Antibacterial activity
Preliminary antibacterial assay results showed that at concentration of 50 μg per disc compounds 3–5 could inhibit the growth of four tested bacterial strains (B. subtilis, E. coli JVC1228, M. luteus UST950701-006, P. nigrifaciens UST010620-005), whereas 1, 2 and 6–8 had no effect on the growth of any tested bacteria. The MIC values of 3–5 were further tested by standard disc diffusion assay. The results (Table 1) showed that 3–5 had medium antibacterial activity against the four tested bacterial strains, and their antibacterial activities against M. luteus UST950701-006 (one marine larval settlement inducing bacterial strain) and P. nigrifaciens UST010620-005 (one marine pathogenic bacterial strain) were stronger than the positive control kanamycin.
As compound 3 showed potent antibacterial activity, the antibacterial activity of 3 against S. onedensis MR-1 biofilm was further evaluated using sterile 24-well polystyrene plates. The results (Figure 4) showed that 3 could completely inhibit the growth of S. onedensis MR-1 at concentration of 3.125 μg ml−1 after 12 h of incubation, whereas as the incubation time increased to 24 h, the pellicle formation recovered well. From the morphological observation, we found that 3 could inhibit the growth of S. oneidensis MR-1 by interfering cells’ regular division, whereas as the culture time increased, cells gradually recovered normal division, and then the bacterium became to grow well.
Antilarval settlement activity
Antifouling activity of 1–8 was evaluated in settlement inhibition assays with B. amphitrite larvae. The results (Figure 5) showed that 1 and 6–8 could strongly inhibit the larvae settlement of B. amphitrite larvae with EC50 values of 6.71, 6.05, 17.9 and 13.67 μg ml−1 respectively, whereas 2 and 3–5 didn’t have antifouling activity at concentration of 25 μg ml−1. No toxicity toward larvae was observed for 1 and 6–8 at the test concentrations of 3.125–100 μg ml−1, which indicated that the LC50/EC50 values of 2 and 6–8 were >14.9, >16.5, <15 and <15, respectively.
Discussion
This study serves as the attempt to find antifouling and antibacterial compounds from gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023 and investigates their possible chemical defense for its host. In the study, we obtained one flavone (1), four secalonic acid analogs (2–5) and three anthraquinones (6–8) from the marine fungal species. Previous studies reported that 3 and 4 had strong cytotoxicity,17, 18 and many attentions have been paid to 3, however, its application was limited because of its teratogenic effect on murine and chick embryo.19, 20 Here, we found that 3–5 had medium antibacterial activity against marine larval settlement inducing bacterium M. luteus, marine pathogenic bacterium P. nigrifaciens, and common bacteria B. subtilis and E. coli, and 3 also showed antibacterial activity against marine bacterium S. onedensis MR-1 biofilm. This was the first report of the antibacterial activity of 3–5 against marine bacteria.
In addition, the new compound 1 showed strong antifouling activity toward B. amphitrite larvae with EC50 value of 6.71 μg ml−1 and low toxicity with LC50/EC50 ratio >14.9. Its structure is similar to the flavones that have strong antilarval settlement activity toward B. amphitrite larvae.21 Anthraquinones 6–8, especially 6 also exhibited strong antifouling activity against B. amphitrite larvae settlement with EC50 <15 μg ml −1, and the LC50/EC50 ratio of 6 was >16.5. Usually, the standard requirement of an efficacy EC50 level for natural antifoulant is 25 μg ml−1 that was established by the US Navy program, and a compound with a LC50/EC50 ratio >15 is often considered as a non-toxic antifouling compound.22 The results indicated that 1 and 6 were potent natural antifoulants. This was the first report of the antifouling activity of 1 and 6–8 against marine biofouling organism’s larvae.
In conclusion, gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023 strain could produce antifouling and antibacterial secondary metabolites that might aid the host gorgonian coral in protection against marine pathogen bacteria, biofouling organisms and other intruders.
Materials and methods
Fungal strain isolation and identification
The sample of gorgonian coral Dichotella gemmacea was collected from the South China Sea, Sanya (18°11′N, 109°25′E), Hainan Province, China. The sample was rinsed three times in sterile seawater to remove transient and loosely attached microbial organisms, and then cut into pieces measuring ca. 1 cm3 and thoroughly homogenized in a sterile mortar with two volumes of sterile seawater. A 10-fold dilution was made and 0.1 ml dilutions were plated on glucose yeast extract peptone (GYP) medium (containing 5 g l−1 glucose, 10 g l−1 yeast extract, 10 g l−1 peptone 5, 30 g l−1 sea salt and 18 g l−1 agar) agar plates. The inoculated plates were incubated at 28 oC for 3 weeks. The fungal isolate SCSGAF 0023 recovered on the media was selected on the basis of their morphological differences based on visible examination of growth characteristics, aerial mycelium, substrate mycelium and diffusible pigments.
Total genomic DNA of fungal isolate SCSGAF 0023 was extracted as described by Lai et al.23 From the genomic DNA, the internal transcribed spacer (ITS) gene sequences were amplified by polymerase chain reaction using primers ITS1(5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGAT ATGC-3′). And the ITS region was sequenced and compared with the GenBank database, which indicated the ITS sequence of fungal isolate SCSGAF 0023 (GenBank access number JN850988) shared a similarity of 99% with Penicillium oxalicum strain F3 (JF793525.1).
Fungal strain cultivation and fermentation
The fungal strain was inoculated in potato dextrose agar (PDA) liquid medium (20 potato, 2 glucose and 3% sea salt), and then cultured in potato dextrose agar liquid nutrient medium under shaking. Twelve-liter cultivations were carried out in 500 ml shake flasks with 150 ml broth and incubated on a rotary shaker (150 r.p.m.) at 28 ° for 6 days.
Extraction and isolation of compounds
The 12 l fermentation broth was filtered through cheesecloth to separate the broth supernatant and mycelia. The broth supernatant was extracted with ethyl acetate, whereas the mycelia was extracted with 80% acetone. The acetone extract was evaporated under reduced pressure to afford an aqueous solution, and then extracted with ethyl acetate. The extracts of broth supernatant and mycelia were combined to give a crude gum (8 g). The crude gum was separated to two parts: methanol soluble and methanol insoluble fractions. The methanol insoluble fraction was repeatedly crystallized from CHCl3/MeOH to yield 3 (200 mg), and its mother liquid was further purified by semi-preparative reversed-phase HPLC (YMC-Pack, ODS S-5 μ 250 × 10 mm i.d., 3 ml min−1), eluting with MeOH/H2O/CH3COOH (v/v/v 90:10:10−4) to yield 5 (10 mg). The methanol soluble fraction was subjected to Rp-18 reverse-phase column chromatography eluting with H2O/MeOH (v/v from 90:10 to 0:100) to give seven fractions (I–VII). Fraction II was subjected to Sephadex LH-20 column chromatography eluting with MeOH to afford four subfractions. Subfraction II-2 was further purified by reversed-phase-HPLC with MeOH-H2O (v/v 58:42) to yield 2 (5 mg), and subfraction II-4 was subjected to Sephadex LH-20 column eluting with CHCl3/MeOH (v/v 1:1), then further purified by preparative TLC plate with CHCl3/MeOH (v/v 8:2) to give 1 (4 mg). Fraction V was chromotographed on a Sephadex LH-20 column, eluting with CHCl3/MeOH (v/v 1:1) to obtain eight subfractions. Subfraction V-2 was further chromotographed on a Sephadex LH-20 column eluting with CHCl3/MeOH (v/v 1:1) to give 6 (5 mg). Subfraction V-7 was repeatedly subjected to silical gel column chromatography using a gradient elution of CHCl3/MeOH to yield 4 (2 mg). Fraction IV was applied to a Sephadex LH-20 column chromatography eluting with CHCl3/MeOH (v/v 1:1) to give five subfractions. Subfraction IV-4 was further purified by reversed-phase-HPLC with CH3CN/H2O (v/v 45:55) to yield 8 (2 mg), and subfraction IV-5 was purified by preparative TLC plate to give 7 (4 mg).
Physical properties of compounds 1 and 2
The structures of compounds 1–8 were determined by spectroscopic analysis. Optical rotations were measured with a Horiba SEAP-300 spectropolarimeter (Horiba Ltd., Kyoto, Japan). UV spectra were measured with a Shimadzu double-beam 210 A spectrophotometer (Shimadzu Corp., Kyoto, Japan) in MeOH solution. 1H, 13C NMR and 2D NMR spectra were recorded on a Bruker AV-500 MHz NMR spectrometer (Bruker Corp., Karlsruhe, Germany) with TMS as internal standard. MS spectral data were obtained on an LCQDECA XP HPLC/MSn spectrometer (Agilent Technologies Inc., San Jose, CA, USA) for ESIMS.
6,8,5′6′-Tetrahydroxy-3′-methylflavone (1): white power; UV (MeOH) λmax: 250, 261, 346 nm; IR (KBr): 3358, 1650, 1602, 1496 cm–1; 1H NMR (CD3OD, 500 MHz) δH: 2.22 (3H, s, H-7′), 6.35 (1H, d, J=1.5 Hz, H-5), 6.28 (1H, d, J=1.5 Hz, H-2′), 6.26 (1H, d, J=1.5 Hz, H-4′), 6.24 (1H, s, H-3), 6.23 (1H, d, J=1.5 Hz, H-7); 13C NMR (CD3OD, 125 MHz) δC: 20.4 (C-7′), 95.0 (C-5), 100.2 (C-7), 101.3 (C-4′), 105.4 (C-4a), 110.2 (C-2′), 112.9 (C-3), 113.2 (C-1′), 140.8 (C-3′), 158.4 (C-5′), 160.3 (C-8a), 161.4 (C-6′), 163.3 (C-8), 166.2 (C-6), 166.3 (C-2), 184.0 (C-4); (+)-ESIMS m/z 301 [M+H]+; (+)-HRESIMS m/z: [M+H]+ 301.0706 (calcd. for C16H13O6, 301.0712).
Paecilin C (2): pale yellow gum; [α]20D -71.35 (c 0.37, CHCl3); UV (MeOH) λmax: 214, 281, 346 nm; IR (KBr): 3422, 1790, 1738, 1650 cm–1; 1H NMR (CDCl3, 500 MHz) δH: 3.29 (2H, d, J=17.5 Hz, H-3), 6.64 (1H, d, J=8.0 Hz, H-6), 7.52 (1H, d, J=8.5 Hz, H-7), 4.82 (1H, d, J=6.5 Hz, H-9), 3.00 (1H, m, H-10), 2.73 (1H, dd, J=8.0, 17 Hz, H-11), 2.52 (1H, dd, J=8.5, 17.5 Hz, H-11), 1.36 (3H, d, J=7.0 Hz, H-13), 3.79 (3H, s, H-15), 11.92 (1H, s, 5-OH), 3.22 (2H, d, J=17.5 Hz, H-3′), 6.63 (1H, d, J=8.0 Hz, H-6′), 7.54 (1H, d, J=8.5 Hz, H-7′), 4.10 (1H, s, H-9′), 2.39 (1H, m, H-10′), 2.67 (1H, dd, J=8.0, 17 Hz, H-11′), 2.46 (1H, m, H-11′), 1.11 (3H, d, J=6.5 Hz, H-13′), 3.77 (3H, s, H-15′), 12.03 (1H, s, 5′-OH); 13C NMR (CDCl3, 125 MHz) δC: 84.5 (C-2), 39.8 (C-3), 195.9 (C-4), 159.2 (C-5), 107.4 (C-6), 140.9 (C-7), 117.4 (C-8), 82.6 (C-9), 33.5 (C-10), 36.7 (C-11), 174.9 (C-12), 14.9 (C-13), 169.1 (C-14), 53.5 (C-15), 107.5 (C-4a), 158.8 (C-8a), 86.8 (C-2′), 39.8 (C-3′), 194.1 (C-4′), 159.2 (C-5′), 107.3 (C-6′), 141.2 (C-7′), 117.8 (C-8′), 76.2 (C-9′), 30.7 (C-10′), 40.2 (C-11′), 174.9 (C-12′), 13.7 (C-13′), 169.1 (C-14′), 53.7 (C-15′), 107.5 (C-4a′), 158.4 (C-8a′); (+)-ESIMS m/z 639 [M+H]+; (+)-HRESIMS m/z: [M+H]+ 639.1701 (calcd. for C32H31O14, 639.1708).
Antibacterial assays
Antibacterial activity of 1−8 was tested against four bacteria (B. subtilis, E. coli JVC1228, Micrococcus luteus UST950701-006, Pseudoalteromonas nigrifaciens UST010620-005) using standard disc diffusion assay. Briefly, first, the tested bacteria were cultured on nutrient agar plates at 37 °C for 1–2 days, then scraped to make cell suspension (108 CFU ml−1) with sterile seawater and sterile water for marine and common bacterial strains, respectively. Forty microliters cell suspension were then mixed with 20 ml corresponding nutrient agar when the agar temperature was kept in 50–60 °C, and then poured into 100 mm sterile Petri plates. Sterile 6 mm diameter circular discs of filter paper were loaded with 50 μg of tested compound dissolved in methanol, evaporated to dryness and then placed onto the bacterial lawn. An additional set of discs with 50 μg kanamycin or penicillin were used as the positive controls. The agar plates were incubated for 18 h at 37 °C, and then the inhibition zones were measured. The experiment was run in three replicates.
The MICs of active compounds 3, 4 and 5 were further determined by disc diffusion assay as the above described. Tested samples were dissolved in methanol at a concentration of 50 μg μl−1, which were diluted to 10 000, 2500, 625, 156, 39, 9.7, 2.4, 0.6 μg ml−1. Five microliters of each concentration were added into each paper disc. An additional set of discs with gradient-based concentrations of penicillin and kanamycin were used as positive controls. MIC value was calculated as the following equation: MIC=Cn+C(n+1)/2. Cn, the lowest concentration at disc with inhibition zones; C(n+1), the highest concentration at disc without inhibition zones.
Antibacterial activity of 3 against Shewanella onedensis MR-1 biofilm was further evaluated using sterile 24-well polystyrene plates.24 S. oneidensis MR-1, a facultative Gram-negative anaerobe with a remarkable respiratory versatility, has been extensively studied for its biofilm development. In here, the strain was grown in 3 ml lysogeny broth to an OD600 of 0.8–1.0. The culture was then diluted to 1:100 in fresh lysogeny broth at 24-well plate, which containing different concentration of 3. The stock solution concentration of 3 was 50 μg ml−1. Double dilution method was used to test the effects of 3 on pellicle formation. The culture in 24-well plate was incubated without shaking at room temperature, and after 12 and 24 h of incubation their pellicles were analyzed by visual inspection.
Larval settlement bioassays
Antifouling activity of compounds 1–8 was evaluated in settlement inhibition assays with laboratory-reared B. amphitrite (Cirripedia) larvae. Larval settlement bioassays were performed using sterile 24-well polystyrene plates as previously reported.25 Briefly, the stock solution of tested samples in DMSO was diluted with autoclaved filtered sea water to concentrations ranging from 3.125 to 100 μg ml−1. About 20 competent larvae were added to each well in 1 ml of the test solution. Wells containing only filtered sea water with DMSO served as the controls. The plates were incubated at 27 °C for 24 h. The percentage of larval settlement was determined by counting the settled, live individuals under a dissecting microscope and expressing the result as a proportion of the total number of larvae in the well. EC50 (inhibits 50% of settlement of B. amphitrite larvae in comparison with the control) and LC50 (refers to the concentration that kills 50% of the test organisms in comparison with the control) levels of active compounds were calculated by using the Excel software program.
Accession codes
References
Coil, J. C. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia). Chem. Rev. 92, 613–631 (1992).
Harvell, C. D. et al. Emerging marine diseases-climate links and anthropogenic factors. Science 285, 1505–1510 (1999).
Shnit-Orland, M. & Kushmaro, A. Coral mucus-associated bacteria: a possible first line of defense. FEMS Microbiol. Ecol. 67, 371–380 (2009).
Zuluaga-Montero, A., Toledo-Hernández, C., Rodríguez, J. A., Sabat, A. M. & Bayman, P. Spatial variation in fungal communities isolated from healthy and diseased sea fans Gorgonia ventalina and seawater. Aquatic. Biology 8, 151–160 (2010).
Wang, Y. N., Shao, C. L., Zheng, C. J., Chen, Y. Y. & Wang, C. Y. Diversity and antibacterial activities of fungi derived from the gorgonian Echinogorgia rebekka from the South China Sea. Mar. Drugs 9, 1379–1390 (2011).
Steyn, P. S. Isolation, structure and absolute configuration of secalonic acid D, toxic metabolite of Penicillium oxalicum. Tetrahedron 26, 51–57 (1970).
Zhang, C. N. et al. Bioassay-guided separation of citreorosein and other oestrogenic compounds from Polygonum cuspidatum. Phytother. Res. 23, 740–741 (2009).
Jiang, T. et al. Chemical constituents from marine fungus penicillium thomii. Acta Pharm. Sinica 37, 271–274 (2002).
Wen, L. et al. Studies on the secondary metabolites and bioactivity of mangrove endophytic fungus Paecilomyces sp.(tree1-7). Chem. Res. Appl. 21, 198–202 (2009).
Kalidhar, S. B. Structural elucidation in anthraquinones using proton NMR glycosylation and alkylation shifts. Phytochemistry 28, 3459–3463 (1989).
Ren, H., Cao, X. L. & Gu, Q. Q. Antitumor metabolites from marine-derived fungus Gliocladium catenulatum T31. Chin. Pharm. J. 45, 1720–1723 (2010).
Shen, C. C., Chang, Y. S. & Ho, L. K. Nuclear magnetic resonance studies of 5,7-dihydroxyflavoids. Phytochemistry 34, 843–845 (1993).
Stochmal, A. et al. Alfalfa (Medicago sativa L.) flavonoids. 1. Apigenin and luteolin glycosides from aerial parts. J. Agric. Food Chem. 49, 753–758 (2001).
Horie, T., Tominaga, H. & Kawamura, Y. Revised structure of a natural flavone from Ageratum conyzoides. Phytochemistry 32, 1076–1077 (1993).
Nishikawa, K. et al. Flavone production in transformed root cultures of Scutellaria baicalensis Georgi. Phytochemistry 52, 885–890 (1999).
Guo, Z. et al. 1H and 13C NMR signal assignments of paecilin A and B, two new chromone derivatives from mangrove endophytic fungus Paecilomyces sp (tree 1-7). Mag. Reson. Chem. 45, 777–780 (2007).
Ren, H., Tian, L., Gu, Q. Q. & Zhu, W. M. Secalonic acid D; A cytotoxic constituent from marine lichen-derived fungus Gliocladium sp T31. Arch. Pharm. Res. 29, 59–63 (2006).
Millot, M., Tomasi, S., Studzinska, E., Rouaud, I. & Boustie, J. Cytotoxic constituents of the Lichen Diploicia canescens. J. Nat. Prod. 72, 2177–2180 (2009).
Reddy, R. V., Eldeib, M. M. & Reddy, C. S. Inhibition of adenylate cyclase in perfusion mouse palate by secalonic acid D. J. Toxicol. Env. Heal 41, 175–185 (1994).
Vesely, D., Vesela, D. & Jelinek, R. Embryotoxicity of T2 toxin and secalonic acid in embryonic chicks varies with the site of administration. Teratology 46, 131–1361 (1992).
Zhou, X. J. et al. Flavone and isoflavone derivatives of terrestrial plants as larval settlement inhibitors of the barnacle Balanus amphitrite. Biofouling 25, 69–76 (2009).
Qian, P. Y., Xu, Y. & Fusetani, N. Natural products as antifouling compounds: recent progress and future perspectives. Biofouling 26, 223–234 (2010).
Lai, X. T. et al. Fungal communities from methane hydrate-bearing deep-sea marine sediments in South China Sea. ISME J. 1, 756–762 (2007).
Liang, Y. L. et al. Pellicle formation in Shewanella oneidensis. BMC Microbiol. 10, 291 (2010).
Qi, S. H., Zhang, S., Qian, P. Y., Xiao, Z. H. & Li, M. Y. Ten new antifouling briarane diterpenoids from the South China Sea gorgonian Junceella juncea. Tetrahedron 62, 9123–9130 (2006).
Acknowledgements
We are grateful to the National Basic Research Program of China (grant 2010CB833803), the National Natural Science Foundation of China (grant 40931160435, 40976090), the Research Supported by the CAS/SAFEA International Partnership Program for Creative Research Teams (grant KZCX2-YW-T001) and the Knowledge Innovation Program of Chinese Academy of Science (grant KSCX2-EW-G-12B) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Bao, J., Sun, YL., Zhang, XY. et al. Antifouling and antibacterial polyketides from marine gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023. J Antibiot 66, 219–223 (2013). https://doi.org/10.1038/ja.2012.110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2012.110
Keywords
This article is cited by
-
Antimicrobial polyketides from Magellan Seamount-derived fungus Talaromyces scorteus AS-242
The Journal of Antibiotics (2023)
-
Anti-neuroinflammatory effect of oxaline, isorhodoptilometrin, and 5-hydroxy-7-(2′-hydroxypropyl)-2-methyl-chromone obtained from the marine fungal strain Penicillium oxalicum CLC-MF05
Archives of Pharmacal Research (2022)
-
Bioactive Indole Diterpenoids and Polyketides from the Marine-Derived Fungus Penicillium javanicum
Chemistry of Natural Compounds (2020)
-
Recycling of Chinese herb residues by endophytic and probiotic fungus Aspergillus cristatus CB10002 for the production of medicinal valuable anthraquinones
Microbial Cell Factories (2019)
-
Biodiversity and antifouling activity of fungi associated with two soft corals from the South China Sea
Archives of Microbiology (2019)