Abstract
Bioactivity-guided fractionation of Streptomyces microflavus neau3 fermentation broth was used to determine the chemical identity of bioactive constituents. The structure of the new compound 1 was determined on the basis of spectroscopic analysis, including 1D and 2D NMR, as well as HRESI-MS, ESI-MS, UV and IR, and compared with the data reported in the literature. The acaricidal and nematocidal activities of the isolated compound 1 and its previously reported analogs 2, 3, 4 and 5 were evaluated. The results demonstrate that compounds 1, 2, 3, 4 and 5, especially compound 1, have potential as antiparasitics with acaricidal and nematocidal activities. These lactones shed new insights into the structural diversity of nemadectin and milbemycin analog libraries that are potentially accessible by combinatorial biosynthesis.
Similar content being viewed by others
Introduction
Microbial metabolites have attracted increasing attention as potential pesticides. They are expected to overcome the resistance and pollution that have accompanied the use of synthetic pesticides.1, 2 Several microbial metabolites, such as the avermectin and milbemycin families, have been proven to be potent preventatives against a variety of pests such as insects and parasites. Moreover, they are the biggest-selling and arguably most effective acaricides and anthelmintics currently available.3, 4, 5 In addition, blasticidin, polyoxin, kasugamycin, validamycin and mildiomycin as fungicides, bialaphos as herbicide, and spinosad as insecticide have been widely used.6 The excellent activities of these compounds suggest that other desirable pesticides will be discovered from microbial metabolites.7, 8, 9, 10
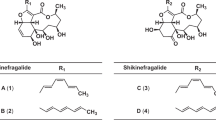
We conducted experiments to screen for new metabolites as pesticides and antiparasitic veterinary drugs or as semi-synthetic intermediates. As a result, a new compound 1 (Figure 1) was isolated from the fermentation broth of Streptomyces microflavus neau3 strain, which was newly obtained from a soil sample. Compound 1 is reminiscent of nemadectin and seco-milbemycin compounds 2, 3, 4 and 5 (Figure 1) produced by S. bingchenggensis.11, 12 At the position of C-25, compound 1 has the same substituent as nemadectin, whereas it is different from compounds 2, 3, 4 and 5. Moreover, nemadectin is a 16-membered lactone, whereas compounds 1, 2, 3, 4 and 5 are six-membered lactones. This paper describes the fermentation, isolation, identification, and acaricidal and nematocidal activities of compound 1. In the course of this work, we also determined the acaricidal and nematocidal activities of compounds 2, 3, 4 and 5 (Figure 1), which we previously reported.11, 12
Results and discussion
Isolation and structural elucidation
Using bioactivity-guided separation of the components in the methanol extract of S. microflavus neau3 fermentation broth, compound 1 was afforded by twice silica gel column chromatography and semi-preparative HPLC. The two seco-milbemycins from the S. bingchenggensis, analogous of compound 1, were obtained using the same approach, demonstrating that the two new seco-milbemycins and corresponding milbemycin β14 were not converted to each other during purification by silica gel column and storage in MeOH extract at room temperature.11
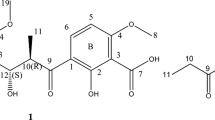
Compound 1 was isolated as colorless oil. Its molecular formula was established as C36H50O7 on the basis of HRESI-MS (m/z 617.3447 [M+Na]+, calcd 617.3449 for C36H50NaO7). The IR spectrum of compound 1 showed absorption bands assignable to the hydroxyl group (3447 cm−1) and an ester carbonyl (1705 cm−1). The 1H NMR (400 MHz, CDCl3) data (Table 1) of compound 1 showed one trans double bond at δ 5.90 (dd, J=14.9, 7.0 Hz) and δ 6.24 (dd, J=14.9, 11.2 Hz), one aromatic methyl at δ 2.24 (s), two vinylic methyls at δ 1.63 (br s) and 1.58 (d, J=0.8 Hz), and four aliphatic methyls at δ 0.79 (d, J=6.9 Hz), 0.93 (d, J=6.6 Hz), 0.98 (d, J=6.6 Hz) and 1.08 (d, J=6.6 Hz). Its 13C NMR and DEPT data (Table 1) revealed an ester carbonyl at δ 165.6 (s), a ketal at δ 99.8 (s), four oxygenated methines, one oxygenated methylene, in addition to 14 sp2 carbons, 5 aliphatic methylenes, 3 aliphatic methines and 7 methyls. Comparison of the 1H and 13C NMR data of compound 1 with those of seco-milbemycin A (compound 2, Figure 1)11 suggested that compound 1 was structurally related to compound 2. The differences between compounds 1 and 2 could be explained by replacement of a methyl carbon and two methylene carbons in compound 2 by three methyl carbons, two methine carbons (one oxygenated) and two olefinic carbons in compound 1. The placement of the seven carbons in compound 1 was revealed by the 1H–1H COSY and HMBC correlations (Figure 2). In the HMBC spectrum of compound 1, the correlation from H-30 to C-23 indicated that the oxygenated methine was C-23. The correlations of H-35, H-36 and H-33 in the 1H–1H COSY spectrum and the HMBC correlations between H-35, H-36 and C-32, and between H-34, C-31 and C-32 connected the remaining six carbons as the 4-methyl-2-pentenyl moiety by analogy with the structure of nemadectin.13, 14 The correlation from H-34 to C-25 in the HMBC spectrum supported the linkage of the 4-methyl-2-pentenyl moiety with C-25 by C-31. Thus, the gross structure of compound 1 was established.
The E configuration of the Δ10 olefin of compound 1 was assigned by the large coupling constant (14.8 Hz) between H-10 and H-11. The NOESY correlation signal between H-29 and H-16 revealed that the double bond of C-14 and C-15 was E. Especially, the evident NOESY signals between H-27 and H-10, and between H-6 and H-9 showed the presence of a trans (E) double bond between C-8 and C-9 (Figure 2).
The configurations of the chiral centers and the 4-methyl-2-pentenyl moiety in compound 1 were assigned by analogy with nemadectin.13 More research is needed in the future to obtain the absolute configuration of compound 1.
We have determined the 16S rDNA sequence of the strain (DQ 924968.1), which produces compound 1. This shared 99.51, 99.24, 99.10 and 99.37% identity with the 16S rDNA sequences of S. microflavus strains HBUM 174011 (FJ532427.1), HBUM 174884 (FJ481633.1), HBUM 174246 (FJ486435.1) and HBUM 174098 (FJ 486343.1), respectively, thereby characterizing strain neau3 as a S. microflavus. Compared with nemadectin producers S. cyaneogriseus ssp. noncyanogenus13 and S. thermoarchaensis,15 S. microflavus neau3 belongs to a distinct species. Although compound 1 is analogous to the seco-milbemycins (compounds 2, 3, 4 and 5, Figure 1)11, 12 produced by S. bingchenggensis, the 16S rDNA sequence of S. microflavus neau3 (DQ 924968.1) only shared 93.81% identity with that of S. bingchenggensis (DQ449953).
Acaricidal and nematocidal activities of the new compound 1 and compounds 2, 3, 4 and 5
Compound 1 from the S. microflavus neau3 and compounds 2, 3, 4 and 5 from S. bingchenggensis were tested for their acaricidal and nematocidal activities. The acaricidal and nematocidal capacities of these compounds were compared with the two known standard acaricides and nematocides, milbemycin A3/A4 and doramectin, in the same assay. As shown in Table 2, although compounds 1, 2, 3, 4 and 5 showed lower acaricidal potencies against adult mites and nematocidal activities against Caenorhabditis elegans than doramectin and milbemycin A3/A4, compound 1 possessed relatively higher acaricidal and nematocidal activities.
Although compound 1 is an analog of nemadectins and milbemycins, we were surprised at these findings. Thus, we determined the bioactivities of compounds 2, 3, 4 and 5, which we previously reported.11, 12 These seco-compounds showed unexpected potencies. It is also reported that Δ2,3-ivermectin-secoester (Figure 1) has a high leishmanicidal activity.16
The discovery of compounds 1, 2, 3, 4 and 5 provides new insights into the biosynthesis of nemadectin and milbemycin. Homologous thioesterases17 from S. microflavus neau3 and S. bingchenggensis presumably are responsible for cyclizations resulting in both 16- and (or) 6-membered lactones. Therefore, this represents new opportunities for macrolide modifications and a new analog design to further expand the size and diversity of nemadectin and milbemycin analog libraries.
Methods
General procedures and reagents
UV spectra were obtained on a Varian CARY 300 BIO spectrophotometer (Varian, Palo Alto, CA, USA). IR spectra were recorded on a Nicolet Magna FT-IR 750 spectrometer (Nicolet, Madison, WI, USA); 1H and 13C NMR spectra were measured with a Bruker DRX-400 (400 MHz for 1H and 100 MHz for 13C) spectrometer (Bruker, Rheinstetten, Germany). Chemical shifts are reported as p.p.m. (δ), using the residual CHCl3 (δH 7.26; δC 77.0) as an internal standard, and coupling constant (J) in Hz. 1H and 13C NMR assignments were supported by 1H–1H COSY, HMQC and HMBC experiments. The ESI-MS and HRESI-MS spectra were taken on a Q-TOF Micro LC-MS-MS mass spectrometer (Micro, Milford, MA, USA). Optical rotation was measured on a Perkin-Elmer 341 polarimeter (Perkin-Elmer, Fremont, CA, USA). Column chromatography was carried out on silica gel (100–200 mesh, Qingdao Haiyang Chemical Group, Qingdao, Shandong, China). Semi-preparative HPLC (Agilent 1100, Zorbax SB-C18, 5 μm, 250 × 9.4 mm i.d.; Agilent, Palo Alto, CA, USA) was further performed to obtain the pure compound. Spots were detected on TLC under UV or by heating after spraying with sulfuric acid–ethanol, 5:95 (v/v). All chemicals used in the study, such as methanol (MeOH), ethyl acetate (EtOAc), petroleum ether (60–90 oC) and acetone, were analytical grade.
Microorganism
The producing organism, S. microflavus neau3, was isolated from a farmland in Laifeng, Hubei province, China, by using selective media humic acid–vitamin medium,18 with 20 μg ml−1 nalidixic acid. S. microflavus neau3 has been deposited at the China General Microbiological Culture Collection Center (Accession No.: CGMCC 3316), Institute of Microbiology, Chinese Academy of Sciences. The 16S rDNA sequence (Accession No.: DQ 924968.1 in GenBank, National Center for Biotechnology Information) was determined.
Fermentation
The strain was maintained on the medium containing glucose (Beijing Ao Bo Xing, Beijing, China) 10 g, maltose (Beijing Ao Bo Xing) 3 g, yeast extract powder (Beijing Ao Bo Xing) 3 g, K2HPO4 3H2O 0.5 g, MgSO4 7H2O 0.5 g, NaCl 0.5 g, KNO3 1 g and agar 20 g in 1.0 l tap water, pH 7.0. The seed medium consisted of glucose (Beijing Ao Bo Xing) 5 g, maltodextrin (Beijing Ao Bo Xing) 5 g, soluble amylum (Beijing Ao Bo Xing) 1 g, yeast autolysate (Beijing Ao Bo Xing) 5 g, beef extract (Beijing Ao Bo Xing) 1 g and casein peptone (Beijing Ao Bo Xing) 1 g in 1.0 l water and pH 7.0. All the media were sterilized at 121 °C for 20 min. Slant culture was incubated for 8–9 days at 28 °C.
A total of 10 ml of sterile water was added to the slant medium. The spores were scraped and transferred to the sterile tube of beading. The spore suspension was then filtered through six layers of sterile filter cheesecloth and adjusted to 107–108 c.f.u. ml−1. In all, 2.0 ml of the spore suspension was inoculated into a 250-ml flask comprising 25 ml of seed medium and incubated at 28 °C for 46 h at 250 r.p.m. Then, 8.0 ml of the culture was transferred into a 1-l Erlenmeyer flask containing 100 ml of the producing medium consisting of glucose (Beijing Ao Bo Xing) 1%, lactose (Beijing Ao Bo Xing) 2.5%, cotton seed powder (Beijing Ao Bo Xing) 2.5% and CaCO3 0.03%, pH 7.0, before sterilization. Fermentation was carried out at 28 °C for 7–8 days on a rotary shaker at 250 r.p.m.
Isolation and purification of compound 1
A total of 30 l broth from 300 producing fermentations was filtered. The resulting cake was washed with water, and both filtrate and wash were discarded. The washed cake was extracted twice for about 24 h with MeOH (10 l). The MeOH extract was evaporated under reduced pressure to approximately 2 l at 45 °C. The resulting concentrate was extracted three times using an equal volume of EtOAc. The combined EtOAc phase was concentrated under reduced pressure to yield 25 g of oily substances. The residual oily substance was chromatographed on silica gel (90 × 5 cm i.d.; Qingdao Haiyang Chemical Group; 100–200 mesh) and eluted with a petroleum ether (60–90 °C)–acetone mixture (95:5, 75:25, 50:50, v/v; each 3 l) of increasing polarity to afford three fractions. Fraction III eluted with a mixture of petroleum ether–acetone (50:50, v/v) had the acaricidal activity against adult mites. This was further separated over silica gel column chromatography (70 × 2.5 cm i.d.) eluted with CHCl3–MeOH (95:5, 90:10 and 85:15, v/v; each 2 l) to give three subfractions. Subfraction II was eluted with a mixture of CHCl3–MeOH (90:10, v/v) having acaricidal activity, and it was separated by semi-preparative HPLC (Agilent 1100, Zorbax SB-C18, 5 μm, 250 × 9.4 mm i.d.; 1.5 ml min−1; 260 nm; Agilent) by eluting with CH3OH–H2O (85:15, v/v) to afford compound 1 (Figure 1; tR 10.2 min, 30 mg).
Compound 1 (C36H50O7)
Seco-nemadectin, colorless oil; [α]25D +45.1 (c. 0.10, EtOH); UV (EtOH) λmax nm (log ɛ): 270 (4.16), 296 (4.03); IR (KBr), νmax cm−1 3447, 2961, 2927, 1705, 1613, 1457, 1383, 1282, 1162, 997; 1H NMR (CDCl3, 400 MHz) δ ; 13C NMR (CDCl3, 100 MHz) δ (see Table 1); ESI-MS m/z 593 [M-H]−; HRESI-MS m/z 617.3447 [M+Na]+, calcd for C36H50NaO7 617.3449.
Acaricidal activity against adult mites and bioactivity-guided fractionation
MeOH solutions containing 0.1% of the individual pure compounds were diluted 10-fold with water containing 0.01% of detergent to prepare 100 μg ml−1 solutions. Then appropriate further dilutions were prepared. Two-spotted spider mites, sensitive to organophosphorus insecticides, were inoculated on the primary leaves of cowpea plants. One day after inoculation, the leaves of the cowpea plants were soaked in the sample solutions for 1–2 s. Then the leaves were kept at 25 °C. After 3 days, the survival of the adult insects was determined with a binocular microscope and the mortality (%) was calculated. Two-spotted spider mites were also used for bioactivity-guided fractionation.
Acaricidal activity against mite eggs
Sample solutions containing 100, 50, 30 and 10 μg ml−1 of individual compounds were prepared. Female adult two-spotted spider mites were allowed to lay eggs on the primary leaves of cowpea plants. The adult mites were removed to obtain test leaves each bearing about 40 eggs. In a manner similar to the preceding example, the test leaves were soaked in the sample solutions for 1–2 s. After 10 days at 25 °C the number of unhatched eggs was counted, and the unhatched egg rates (%) were calculated.
Nematocidal activity
MeOH solutions containing 0.1% of individual compounds were diluted 10-fold with water to prepare solutions containing 100 μg ml−1. Then appropriate amounts of the solutions were added to 1-l portions of an aqueous suspension containing living nematodes C. elegans. The mixtures were left at 25 °C for 15 h after shaking. The number of nematodes that were immobilized and the total number of nematodes tested were counted under a stereoscopic microscope. Immobilized rates (%) against the total number of tested nematodes were calculated.
References
Tanaka, Y. & Omura, S. Agroactive compounds of microbial origin. Annu. Rev. Microbiol. 47, 57–87 (1993).
Saxena, S. & Pandey, A. K. Microbial metabolites as eco-friendly agrochemicals for the next millennium. Appl. Microbiol. Biotechnol. 55, 395–403 (2001).
Montesinos, E. Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 6, 245–252 (2003).
Fox, L. M. Ivermectin: uses and impact 20 years on. Curr. Opin. Infect. Dis. 19, 588–593 (2006).
Omura, S. Ivermectin: 25 years and still going strong. Int. J. Antimicrob. Agents 31, 91–98 (2008).
Wheeler, W. B. Role of research and regulation in 50 years of pest management in agriculture. Prepared for the 50th anniversary of the Journal of Agricultural and Food Chemistry. J. Agric. Food Chem. 50, 4151–4155 (2002).
Dayan, F. E., Cantrell, C. L. & Duke, S. O. Natural products in crop protection. Bioorg. Med. Chem. 17, 4022–4034 (2009).
Copping, L. G. & Duke, S. O. Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 63, 524–554 (2007).
Demain, A. L. & Sanchez, S. Microbial drug discovery: 80 years of progress. J. Antibiot. 62, 5–16 (2009).
Thompson, G. D., Dutton, R. & Sparks, T C Spinosad—a case study: an example from a natural products discovery programme. Pest. Manag. Sci. 56, 696–702 (2000).
Xiang, W. S., Wang, J. D. & Wang, X. J. New seco-milbemycins from Streptomyces bingchenggensis: fermentation, isolation and structure elucidation. J. Antibiot. 61, 27–32 (2008).
Wang, X. J., Wang, J. D. & Xiang, W. S. Three new milbemycin derivatives from Streptomyces bingchenggensis. J. Asian Nat. Prod. Res. 11, 597–603 (2009).
Carter, G. T., Nietsche, J. A. & Borders, D. B. Strucure determination of LL-F28249 α, β,γ and λ, potent antiparasitic macrolides from Streptomyces cyaneogriseus ssp. Noncyanogenus. J. Chem. Soc. Chem. Commun. 109, 402–404 (1987).
Carter, G. T. et al. LL-F28249 antibiotic complex: a new family of antiparasitic macrocyclic lactones. Isolation, characterization and structures of LL-F28249 α, β, γ and λ. J. Antibiot. 41, 519–529 (1988).
Ward, J. B., Noble, H. M., Porter, N., Fletton, R. A. & Noble, D. Antibiotic compounds and their preparation E.P. 2,166,436A, 8 May (1986).
Santos, A. R. et al. Ivermectin-derived leishmanicidal compounds. Bioorg. Med. Chem. 17, 496–502 (2009).
Ikeda, H., Nonomiya, T., Usami, M., Ohta, T. & Omura, S. Organization of the biosynthetic gene cluster for the polyketide anthelmintic macrolide avermectin in Streptomyces avermitilis. Proc. Natl Acad. Sci. USA 21, 526–531 (2003).
Hayakawa, M. Studies on the isolation and distribution of rare actinomycetes in soil. Actinomycetol. 22, 12–19 (2008).
Acknowledgements
This work was supported by the National Key Project for Basic Research (No. 2010CB126102), the National Natural Science Foundation of China (No. 30571234 and 30771427) and the National Key Technology R&D Program (No. 2006BAD31B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiang, WS., Wang, JD., Wang, M. et al. New nemadectin congener from Streptomyces microflavus neau3: fermentation, isolation, structure elucidation and biological activities. J Antibiot 63, 171–175 (2010). https://doi.org/10.1038/ja.2010.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2010.12
Keywords
This article is cited by
-
An appraisal of natural products active against parasitic nematodes of animals
Parasites & Vectors (2019)
-
Designed biosynthesis of 25-methyl and 25-ethyl ivermectin with enhanced insecticidal activity by domain swap of avermectin polyketide synthase
Microbial Cell Factories (2015)