Abstract
Background/Objectives:
Obese subjects have increased number of enlarged fat cells that are reduced in size but not in number in post-obesity. We performed DNA methylation profiling in fat cells with the aim of identifying differentially methylated DNA sites (DMS) linked to adipose hyperplasia (many small fat cells) in post-obesity.
Subjects/Methods:
Genome-wide DNA methylation was analyzed in abdominal subcutaneous fat cells from 16 women examined 2 years after gastric bypass surgery at a post-obese state (body mass index (BMI) 26±2 kg m–2, mean±s.d.) and from 14 never-obese women (BMI 25±2 kg m–2). Gene expression was analyzed in subcutaneous adipose tissue from nine women in each group. In a secondary analysis, we examined DNA methylation and expression of adipogenesis genes in 15 and 11 obese women, respectively.
Results:
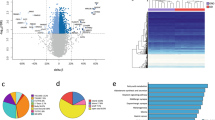
The average degree of DNA methylation of all analyzed CpG sites was lower in fat cells from post-obese as compared with never-obese women (P=0.014). A total of 8504 CpG sites were differentially methylated in fat cells from post-obese versus never-obese women (false discovery rate 1%). DMS were under-represented in CpG islands and surrounding shores. The 8504 DMS mapped to 3717 unique genes; these genes were over-represented in cell differentiation pathways. Notably, 27% of the genes linked to adipogenesis (that is, 35 of 130) displayed DMS (adjusted P=10−8) in post-obese versus never-obese women. Next, we explored DNA methylation and expression of genes linked to adipogenesis in more detail in adipose tissue samples. DMS annotated to adipogenesis genes were not accompanied by differential gene expression in post-obese compared with never-obese women. In contrast, adipogenesis genes displayed differential DNA methylation accompanied by altered expression in obese women.
Conclusions:
Global CpG hypomethylation and over-representation of DMS in adipogenesis genes in fat cells may contribute to adipose hyperplasia in post-obese women.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Accession codes
References
Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351: 2683–2693.
Plecka Ostlund M, Marsk R, Rasmussen F, Lagergren J, Naslund E . Morbidity and mortality before and after bariatric surgery for morbid obesity compared with the general population. Br J Surg 2011; 98: 811–816.
Laurino Neto RM, Herbella FA, Tauil RM, Silva FS, de Lima SE Jr . Comorbidities remission after Roux-en-Y gastric bypass for morbid obesity is sustained in a long-term follow-up and correlates with weight regain. Obes Surg 2012; 22: 1580–1585.
Sethi JK, Vidal-Puig AJ . Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res 2007; 48: 1253–1262.
Lofgren P, Andersson I, Adolfsson B, Leijonhufvud BM, Hertel K, Hoffstedt J et al. Long-term prospective and controlled studies demonstrate adipose tissue hypercellularity and relative leptin deficiency in the postobese state. J Clin Endocrinol Metab 2005; 90: 6207–6213.
Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 2010; 59: 105–109.
Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O et al. Dynamics of fat cell turnover in humans. Nature 2008; 453: 783–787.
Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS . Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol 2009; 5: 401–408.
Bird A . DNA methylation patterns and epigenetic memory. Genes Dev 2002; 16: 6–21.
Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA 2005; 102: 10604–10609.
Ronn T, Volkov P, Davegardh C, Dayeh T, Hall E, Olsson AH et al. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet 2013; 9: e1003572.
Nilsson E, Jansson PA, Perfilyev A, Volkov P, Pedersen M, Svensson MK et al. Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes 2014; 63: 2962–2976.
Dick KJ, Nelson CP, Tsaprouni L, Sandling JK, Aissi D, Wahl S et al. DNA methylation and body-mass index: a genome-wide analysis. Lancet 2014; 383: 1990–1998.
Reinius LE, Acevedo N, Joerink M, Pershagen G, Dahlen SE, Greco D et al. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One 2012; 7: e41361.
Kovacikova M, Sengenes C, Kovacova Z, Siklova-Vitkova M, Klimcakova E, Polak J et al. Dietary intervention-induced weight loss decreases macrophage content in adipose tissue of obese women. Int J Obes (Lond) 2011; 35: 91–98.
Kolaczynski JW, Morales LM, Moore JH Jr., Considine RV, Pietrzkowski Z, Noto PF et al. A new technique for biopsy of human abdominal fat under local anaesthesia with Lidocaine. Int J Obes Relat Metab Disord 1994; 18: 161–166.
Lofgren P, Hoffstedt J, Naslund E, Wiren M, Arner P . Prospective and controlled studies of the actions of insulin and catecholamine in fat cells of obese women following weight reduction. Diabetologia 2005; 48: 2334–2342.
Dayeh T, Volkov P, Salo S, Hall E, Nilsson E, Olsson AH et al. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet 2014; 10: e1004160.
Bibikova M, Lin Z, Zhou L, Chudin E, Garcia EW, Wu B et al. High-throughput DNA methylation profiling using universal bead arrays. Genome Res 2006; 16: 383–393.
Du P, Kibbe WA, Lin SM . Lumi: a pipeline for processing Illumina microarray. Bioninformatics 2008; 24: 1547–1548.
Zhang B, Kirov S, Snoddy J . WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 2005; 33: W741–W748.
Smyth GK . Limma: linear models for microarray data. In: Bioinformatics and Computational Biology Solutions Using R and Bioconductor Edited by Gentleman RCV, Dudoit S, Irizarry R, Huber W Springer: New York, 2005; 397–420.
Woldt E, Matz RL, Terrand J, Mlih M, Gracia C, Foppolo S et al. Differential signaling by adaptor molecules LRP1 and ShcA regulates adipogenesis by the insulin-like growth factor-1 receptor. J Biol Chem 2011; 286: 16775–16782.
Du B, Cawthorn WP, Su A, Doucette CR, Yao Y, Hemati N et al. The transcription factor paired-related homeobox 1 (Prrx1) inhibits adipogenesis by activating transforming growth factor-beta (TGFbeta) signaling. J Biol Chem 2013; 288: 3036–3047.
Grunberg JR, Hammarstedt A, Hedjazifar S, Smith U . The novel secreted adipokine WNT1-inducible signaling pathway protein 2 (WISP2) is a mesenchymal cell activator of canonical WNT. J Biol Chem 2014; 289: 6899–6907.
Masson O, Chavey C, Dray C, Meulle A, Daviaud D, Quilliot D et al. LRP1 receptor controls adipogenesis and is up-regulated in human and mouse obese adipose tissue. PLoS One 2009; 4: e7422.
Jung JS, Jee MK, Cho HT, Choi JI, Im YB, Kwon OH et al. MBD6 is a direct target of Oct4 and controls the stemness and differentiation of adipose tissue-derived stem cells. Cell Mol Life Sci 2013; 70: 711–728.
Isakson P, Hammarstedt A, Gustafson B, Smith U . Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes 2009; 58: 1550–1557.
Olivier M, Tanck MW, Out R, Villard EF, Lammers B, Bouchareychas L et al. Human ATP-binding cassette G1 controls macrophage lipoprotein lipase bioavailability and promotes foam cell formation. Arterioscler Thromb Vasc Biol 2012; 32: 2223–2231.
Eto M, Shindou H, Shimizu T . A novel lysophosphatidic acid acyltransferase enzyme (LPAAT4) with a possible role for incorporating docosahexaenoic acid into brain glycerophospholipids. Biochem Biophys Res Commun 2014; 443: 718–724.
Nouws J, Nijtmans L, Houten SM, van den Brand M, Huynen M, Venselaar H et al. Acyl-CoA dehydrogenase 9 is required for the biogenesis of oxidative phosphorylation complex I. Cell Metab 2010; 12: 283–294.
Doco-Fenzy M, Leroy C, Schneider A, Petit F, Delrue MA, Andrieux J et al. Early-onset obesity and paternal 2pter deletion encompassing the ACP1, TMEM18, and MYT1L genes. Eur J Hum Genet 2014; 22: 471–479.
Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science 2013; 341: 275–278.
Sun XY, Hayashi Y, Xu S, Kanou Y, Takagishi Y, Tang YP et al. Inactivation of the Rcan2 gene in mice ameliorates the age- and diet-induced obesity by causing a reduction in food intake. PLoS One 2011; 6: e14605.
Broberg K, Zhang M, Strombeck B, Isaksson M, Nilsson M, Mertens F et al. Fusion of RDC1 with HMGA2 in lipomas as the result of chromosome aberrations involving 2q35-37 and 12q13-15. Int J Oncol 2002; 21: 321–326.
Loos RJ . Genetic determinants of common obesity and their value in prediction. Best Pract Res Clin Endocrinol Metab 2012; 26: 211–226.
Grundberg E, Meduri E, Sandling JK, Hedman AK, Keildson S, Buil A,B et al. Global analysis of DNA methylation variation in adipose tissue from twins reveals links to disease-associated variants in distal regulatory elements. Am J Hum Genet 2013; 93: 876–890.
Barres R, Osler ME, Yan J, Rune A, Fritz T, Caidahl K et al. Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab 2009; 10: 189–198.
Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 2012; 15: 405–411.
Musri MM, Parrizas M . Epigenetic regulation of adipogenesis. Curr Opin Clin Nutr Metab Care 2012; 15: 342–349.
Chia DJ . MINIREVIEW: Mechanisms of growth hormone-mediated gene regulation. Mol Endocrinol 2014; 28: 1012–1025.
Lou H, Le F, Zheng Y, Li L, Wang L, Wang N et al. Assisted reproductive technologies impair the expression and methylation of insulin-induced gene 1 and sterol regulatory element-binding factor 1 in the fetus and placenta. Fertility and sterility 2014; 101: 974–980 e972.
Diakiw SM, Perugini M, Kok CH, Engler GA, Cummings N, To LB et al. Methylation of KLF5 contributes to reduced expression in acute myeloid leukaemia and is associated with poor overall survival. Br J Haematol 2013; 161: 884–888.
Borland MG, Krishnan P, Lee C, Albrecht PP, Shan W, Bility MT et al. Modulation of aryl hydrocarbon receptor (AHR)-dependent signaling by peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) in keratinocytes. Carcinogenesis 2014; 35: 1602–1612.
Tandon M, Gokul K, Ali SA, Chen Z, Lian J, Stein GS . P et al. Runx2 mediates epigenetic silencing of the bone morphogenetic protein-3B (BMP-3B/GDF10) in lung cancer cells. Mol Cancer 2012; 11: 27.
Sheen-Chen SM, Lin CR, Chen KH, Yang CH, Lee CT, Huang HW et al. Epigenetic histone methylation regulates transforming growth factor beta-1 expression following bile duct ligation in rats. J Gastroenterol 2013; 49: 1285–1297.
Lachance G, Uniacke J, Audas TE, Holterman CE, Franovic A, Payette J et al. DNMT3a epigenetic program regulates the HIF-2alpha oxygen-sensing pathway and the cellular response to hypoxia. Proc Natl Acad Sci USA 2014; 111: 7783–7788.
Zhang Q, Ramlee MK, Brunmeir R, Villanueva CJ, Halperin D, Xu F . Dynamic and distinct histone modifications modulate the expression of key adipogenesis regulatory genes. Cell Cycle 2012; 11: 4310–4322.
Jones PA . Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 2012; 13: 484–492.
Acknowledgements
We thank Patrik Muller for excellent technical assistance with the DNA methylation assay. This study was supported by the SRP Diabetes program at Karolinska Institutet, the Swedish research council, the Erling-Persson Family Foundation, Novo Nordic Foundation, EASD/Eli-Lilly Foundation, the Swedish Diabetes Foundation, and the EU/EFPIA Innovative Medicines Initiative Joint Undertaking (EMIF grant no 115372).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Dahlman, I., Sinha, I., Gao, H. et al. The fat cell epigenetic signature in post-obese women is characterized by global hypomethylation and differential DNA methylation of adipogenesis genes. Int J Obes 39, 910–919 (2015). https://doi.org/10.1038/ijo.2015.31
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.31
This article is cited by
-
A negative feedback loop between TET2 and leptin in adipocyte regulates body weight
Nature Communications (2024)
-
Integrative genomic analyses in adipocytes implicate DNA methylation in human obesity and diabetes
Nature Communications (2023)
-
Recent progress in epigenetics of obesity
Diabetology & Metabolic Syndrome (2022)
-
Effect of excess weight and insulin resistance on DNA methylation in prepubertal children
Scientific Reports (2022)
-
Clinical epigenetics and restoring of metabolic health in severely obese patients undergoing batriatric and metabolic surgery
Updates in Surgery (2022)