Abstract
Background:
Diet is a key modifiable factor in the prevention and treatment of the metabolic syndrome. However, few studies have examined the prospective association between time-of-day of nutrient intake and the metabolic syndrome.
Objective:
To examine the association between time-of-day and nutrient composition of eating occasions and the long-term development of metabolic syndrome in the Medical Research Council (MRC) National Survey of Health and Development (NSHD; 1946 British birth cohort).
Methods:
The analysis comprised 1488 survey members who completed at least 3 days of estimated diet records at age 43 years (1989) and for whom data on metabolic syndrome at age 53 years (1999) were available. Dietary records were divided into seven meal slots: breakfast, mid-morning, lunch, mid-afternoon, dinner, late evening and extras. Metabolic syndrome was defined by the criteria of the adult treatment panel (ATPIII8), and was modified to include glycosylated haemoglobin instead of fasting glucose. Associations between time-of-day of nutrient intake at age 43 years and prevalence of metabolic syndrome at age 53 years were assessed using multivariate nutrient density logistic models after adjustment for sex, social class, smoking status, region, alcohol intake and recreational physical activity.
Results:
There were 390 cases of metabolic syndrome at age 53 years. Substituting 5% of energy from carbohydrate for a similar amount of energy from fat at breakfast (odds ratio=0.93; 95% confidence interval=0.89–0.98; P=0.002) and mid-morning at age 43 years (odds ratio=0.96; 95% confidence interval=0.93–0.99; P=0.011) was associated with lower odds of the metabolic syndrome at age 53 years. Carbohydrate intake at breakfast or mid-morning was particularly protective against abdominal obesity (P⩽0.001). Increasing carbohydrate intake at breakfast while simultaneously decreasing fat intake was also negatively related to triacylglycerols (P⩽0.001).
Conclusions:
Increasing carbohydrate intake in the morning while simultaneously reducing fat intake could be protective against long-term development of the metabolic syndrome and its components.
Similar content being viewed by others
Introduction
The metabolic syndrome is a cluster of risk factors, including abdominal obesity, glucose intolerance, hypertension and dyslipidaemia, that share insulin resistance as a common underlying pathophysiological disturbance.1 This constellation of metabolic abnormalities has been shown to be related to heightened risk of type 2 diabetes, cardiovascular disease and all-cause mortality.2, 3 In recent years, the prevalence of the metabolic syndrome has increased in parallel to the epidemic rise in obesity,4 with prevalence exceeding 20% of the population in some developed countries.5 This rise in metabolic syndrome prevalence carries important clinical implications as the metabolic syndrome accounts for 12–17% and 30–52% of the population-attributable risk for cardiovascular disease and diabetes, respectively.6
Multiple environmental and genetic factors have been implicated in the aetiology of the metabolic syndrome.7 Diet is one of the key modifiable risk factors that may be involved in the prevention and treatment of metabolic syndrome.1 Several epidemiological studies have described associations between single foods,8, 9 individual nutrients (for example, fat, fibre),10, 11 dietary patterns12, 13 and the metabolic syndrome or its individual components. Current evidence also suggests that the time, nutrient composition,14, 15, 16 frequency17, 18 and regularity of meals17, 19 can affect insulin resistance, obesity, dyslipidaemia or the metabolic syndrome independently of traditional risk factors. In mice, high-fat intake in the evening has been shown to induce hyperinsulinaemia, hyperglycaemia, leptinaemia and weight gain.14 Similarly, consumption of a high-fibre or high-carbohydrate breakfast, as opposed to a high-protein, high-fat breakfast, has been linked to lower daily energy intake and body mass index (BMI).16, 20 In shift workers, changes in the distribution of meals predict the development of metabolic syndrome.21 Eating breakfast appears to be protective, whereas higher energy intake in the evening promotes the development of metabolic syndrome.22 This effect is thought to be achieved through circadian misalignment, a condition that induces metabolic changes that mimic the metabolic syndrome.21
Circadian rhythms modulate several physiological and metabolic processes in the human body, including glucose homoeostasis and insulin secretion.23, 24 Glucose tolerance and insulin sensitivity decrease progressively through the day reaching a nadir at night.23, 24 Similarly, the postprandial concentration of triacylglycerols is higher at night compared with daytime.25 By contrast, blood pressure shows a surge in the morning followed by a decline at night.26 Based on this variability in metabolism, it could be hypothesised that there are physiological windows of time when consumption of certain nutrients may be favoured. Consistent with this, short-term trials have demonstrated that nutrient composition of meals can influence glucose, insulin and triacylglycerol responses,27, 28 with higher responses being observed after a night-time meal compared with a day-time meal.29
To our knowledge, no epidemiological study has investigated the association between time-of-day and nutrient composition of eating occasions and the metabolic syndrome and its individual components, and there are no published longitudinal associations. The present study aimed to examine these associations.
Materials and methods
Study population
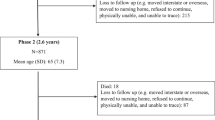
The study population were members of the Medical Research Council (MRC) National Survey of Health and Development (NSHD), a socially stratified longitudinal cohort of 2815 men and 2547 women born in England, Scotland and Wales in 1 week of March 1946. Survey members have been followed-up from birth to age 65 years (2011) during which data on lifestyle, education, occupation and health have been collected. The current analyses included data from 1488 survey members who completed at least 3 days of diet records at age 43 years and for whom information on the metabolic syndrome was available at age 53 years. The population interviewed at ages 43 and 53 years were still representative in most respects of the native-born population of similar age.30 The present study was conducted according to the guidelines laid down in the declaration of helsinki. Ethical approval was obtained from the North Thames Multicentre Research Ethics Committee. NSHD data can be obtained from the NSHD Data Archive at http://www.nshd.mrc.ac.uk/data.aspx.
Dietary assessment
All the food and drink consumed at home and away were recorded in 5-day estimated diet records. Detailed guidance notes and photographs were provided at the beginning of the dietary records to assist cohort members in describing portion sizes using household measures. Each diary day comprised of eight meal slots labelled: prebreakfast, breakfast, mid-morning, lunch, mid-afternoon, dinner, late evening and extras. The ‘extras’ meal slot was included to permit cohort members to record any food or drink items that may have been missed in previous meal slots. For the purpose of our analysis, the prebreakfast and breakfast meal slots were merged, as only 3% of the population reported eating >100 kcal during the prebreakfast meal slot. Diet records at age 43 years were coded at the MRC Dunn Nutrition Unit using the in-house programme Diet In Data Out (DIDO) and nutrient intakes were calculated using the in-house suite of programs based on McCance and Widdowson’s The Composition of Foods, taking into account food composition and portion sizes appropriate to the period of consumption.31, 32
Metabolic syndrome definition
The metabolic syndrome was defined based on the adult treatment panel (ATPIII8) 2001 cutoffs.1 The definition was modified to include glycosylated haemoglobin (>5.8% for both men and women or use of diabetes medication) instead of fasting glucose, as data on this measure were not available. Study members were classified as having the metabolic syndrome if they fulfilled ⩾3 of the following criteria: (1) high waist circumference (>102 cm for men, >88 cm for women); (2) triacylglycerol ⩾1.7 mmol l−1 (3); low HDL cholesterol (<1.036 mmol l−1 for men, <1.295 mmol l−1 for women); (4) high blood pressure (systolic blood pressure ⩾130 or diastolic blood pressure ⩾85 mm Hg or the use of antihypertensive medication); and (5) glycosylated haemoglobin above the top gender-specific quarter of the distribution (>5.8% for both men and women) or the use of diabetes medication. Measurements were taken during a home visit by trained research nurses. Waist circumference was measured to the nearest 1 mm midway between the costal margin and the iliac crest and in line with the mid-axilla. The measurement was taken with a tape in mid-expiration with survey members standing straight with their feet together and arms hanging on the side. Sitting blood pressure was measured after 5 min of rest using an Omron HEM-705 automated digital oscillometric sphygmomanometer (Omron, Tokyo, Japan). Duplicate measurements were obtained, but only the second measurement was used in the definition of the metabolic syndrome to reduce the effect of white coat hypertension. Non-fasting venous blood samples were drawn into serum tubes for measurement of triacylglycerols, high-density cholesterol and into EDTA tubes for measurement of glycosylated haemoglobin. Details of the assays used to analyse these samples have been described elsewhere.33
Covariates
Potential confounders were behavioural factors that have been related to metabolic syndrome. Occupational social class at age 43 years was defined as non-manual (managerial, professional, skilled professional ancillaries and service providers) or manual (skilled, non-skilled and agricultural workers). Smoking status at age 53 years was categorised as current smoker, ex-smoker or non-smoker. Region of residence was defined as: (1) Scotland and the North of England; (2) Eastern, Midlands and Wales; and (3) London and the South of England. Physical activity was estimated as the frequency of recreational activities assessed by administered questionnaire. Four categories of frequency of sports or recreational activities were defined: none, less than once a week, 1–2 times a week or 3 or more times a week. Alcohol consumption was estimated from dietary records and categorised as: none, 1–2 units per day or 3 or more units per day. One unit was equivalent to 8 g of alcohol.
Statistical analysis
Differences in socio-demographic characteristics and nutrient intakes between survey members with or without the metabolic syndrome were compared using t-test for continuous variables or χ2 tests for categorical variables.
Associations between nutrient composition of eating occasions and the metabolic syndrome were assessed using multivariate nutrient density models,34 which evaluated the effect of substituting 5% of energy from carbohydrate for a similar amount of energy from fat (model 1). The odds ratio for these models can be interpreted as the effect of increasing energy intake from one nutrient, while simultaneously reducing energy intake from the nutrient that is excluded from the model. Thus, in a model that includes energy, percentage energy from carbohydrate and percentage energy from protein, the odds ratio is interpreted as the effect of isocaloric substituting carbohydrate for the same amount of energy from fat. The advantage of using nutrient density models is that nutrients are expressed as the percentage of energy intake. This approach allows findings to be directly translated to dietary recommendations that are most often expressed in this way.34
In addition to including energy intake and macronutrient densities, we also adjusted for sex, occupational social class and region in model 1. In model 2, we additionally adjusted for smoking status, alcohol intake and recreational physical activity. We chose not to adjust for BMI because of its strong correlation with waist circumference. According to Skilton et al.,35 adjusting for BMI, in this case, will produce findings that address the association between macronutrient composition and the non-obesity components of the metabolic syndrome. We also conducted analyses controlling for energy intake at each eating occasion instead of daily energy intake. These models examined whether the association between time-of-day of nutrient intake and the metabolic syndrome was independent of energy intake at the eating occasion.
Sensitivity analyses were performed to determine the effect of influential points. Points with large residual and leverage were identified and subsequently removed from models. Removing influential points did not alter the findings of the initial models. Analyses were also repeated using multiple imputations to handle missing covariate information.
Additional regression models were run using the individual components of the metabolic syndrome as the outcome variables in the logistic regression. We employed similar models to the ones described above but focused only on the eating occasions that showed associations with the metabolic syndrome. This was done to investigate whether all components were influenced similarly.
All data were analysed using Predictive Analytics SoftWare version 18 (SPSS Inc., Chicago, IL, USA). Multiple imputations were conducted in STATA release 11 (Stata Corp., College Station, TX, USA). Significance level was set at P<0.01 to account for multiple testing.
Results
Missing data
Out of the 2280 cohort members who had dietary data at age 43 years, 1588 had data for the metabolic syndrome at age 53 years and 692 had missing data for the metabolic syndrome at age 53 years. Those with missing data for metabolic syndrome were more likely to be females (P=0.036), have a manual occupation (P=0.005) and consume more than two units of alcohol per day at age 43 years (P<0.001) compared with cohort members who had data on the metabolic syndrome. Cohort members with missing data also obtained more energy from alcohol at age 43 years between lunch and dinner (P<0.001) and had lower energy intake from fat (P<0.05). They also reported higher alcohol intake (P=0.027) and lower carbohydrate intake in late evening (P=0.05).
Of the 1588 individuals who had data for metabolic syndrome, 99 had missing data for occupational social class and 1 had missing data for region. Regression coefficients after multiple imputations remained similar and no differences in conclusions were observed, hence only complete case analysis is reported.
General characteristics of the population
General characteristics of the sample are shown in Table 1. Of the initial 1488 survey members interviewed at age 43 years, 390 had the metabolic syndrome at age 53 years. Men, ex-smokers, individuals with lower recreational activity, manual occupation or those residing in Scotland were more likely to have the metabolic syndrome. Survey members with the metabolic syndrome also had higher BMI at ages 36, 43 and 53 years than those without the metabolic syndrome and differed in terms of their nutrient intake (Table 1). Individuals with the metabolic syndrome had lower mean daily calcium, vitamin C and non-starch polysaccharide intakes. They also obtained a greater proportion of daily energy from protein and alcohol, while energy intake from carbohydrate was lower compared with cohort members without the metabolic syndrome.
Distribution of energy and nutrient intake
Individuals with the metabolic syndrome obtained a greater amount of energy from fat at breakfast (Table 2). They also had lower carbohydrate intake between breakfast and lunch. Compared with survey members without the metabolic syndrome, individuals with the metabolic syndrome obtained a greater proportion of energy from protein at dinner.
Association between time and nutrient composition of eating occasions and the metabolic syndrome and its individual components
In energy substitution models, increasing energy intake from carbohydrate at the expense of a similar amount of energy from fat at breakfast and at mid-morning was associated with decreased prevalence of the metabolic syndrome (Table 3). These associations were hardly altered and remained significant after adjustment for potential confounders. Adjusting for energy intake of the eating occasions produced similar findings to models that adjusted for total energy intake (data not shown).
The metabolic variable that was most influenced by the time and nutrient composition of eating occasions was waist circumference. Increasing energy intake from carbohydrate at breakfast or mid-morning, while simultaneously decreasing energy intake from fat (Table 4), was associated with decreased prevalence of abdominal obesity. Substituting 5% of energy from carbohydrate for a similar amount of energy from fat at breakfast was related to lower odds of having high triacylglycerols. Obtaining a greater percentage of energy over the day from carbohydrate, while simultaneously decreasing energy intake from fat, was also related to lower prevalence of abdominal obesity. Higher carbohydrate intake over the day was also protective against elevated glycosylated haemoglobin. Greater energy intake over the day from protein at the expense of fat, on the other hand, was positively related to abdominal obesity (odds ratio=1.27; 95% confidence interval=1.11–1.45; P<0.001). No associations between nutrient composition of selected eating occasions and prevalence of hypertension were observed.
Discussion
The present study shows that the time-of-day and macronutrient composition of eating occasions predicts the development of the metabolic syndrome, and its individual components, independently of total energy intake and socioeconomic and other behavioural risk factors in a nationally representative birth cohort. These findings are consistent with previous epidemiological evidence on shift workers, reporting an association between breakfast composition and BMI and short-term trials showing an association between time-of-day of energy intake and the metabolic syndrome.21, 22, 29, 36, 37, 38, 39, 40, 41 Our study extends current evidence by demonstrating that the nutrient composition of different eating occasions predicts metabolic syndrome development. To our knowledge, this is the first prospective study that examines the association between the metabolic syndrome and nutrient composition of all eating occasions, as opposed to focusing on breakfast or night meals only. Our findings suggest that the morning period, in particular, is most critical in terms of maintaining optimum metabolism. Indeed, previous epidemiological evidence has shown that consumption of breakfast, particularly high-carbohydrate breakfast, is related to lower daily energy intake, reduced BMI, improved nutrient intake16, 42 and lower serum cholesterol concentrations.43 The amount of carbohydrate eaten at breakfast has also been reported to modulate glucose, insulin and free fatty acid responses at subsequent meals.44 In mice, consumption of a high-fat diet at the end of the active phase, the equivalent of evening eating in humans, impairs metabolic plasticity and increases daily energy intake leading to weight gain, increased adiposity, impaired glucose intolerance, hyperinsulinaemia, hypertriglyceridemia and hyperleptinaemia.14 Although the mechanism by which time-of-day of nutrient intake influences insulin resistance and other metabolic parameters remains to be determined; these effects are likely to be mediated by circadian rhythms.
Circadian rhythms regulate several physiological processes that influence glucose, cholesterol and blood pressure homoeostasis. Insulin secretion is generally 50% higher at night.24 However, this rise in insulin secretion is counterbalanced by an increase in insulin clearance, which then translates into lower nocturnal insulin sensitivity.23 Consistent with this, glucose tolerance decreases progressively through the day reaching a nadir at night.45 These diurnal changes in metabolism may explain why consumption of a standard meal at lunch or at night produces greater glucose and insulin responses compared with when the same meal is consumed at breakfast or lunch, respectively.29, 46 This, in turn, may explain why evening or night eating is often associated with weight gain and obesity.47 Substrate oxidation also varies according to time-of-day with lower fat oxidation and higher carbohydrate oxidation being observed in the morning.37 In a series of randomised controlled trials, Frape et al.27 found that small differences in the fat composition of breakfast produce profound effects on diurnal levels of circulating non-esterified fatty acids, which can adversely affect glucose tolerance up to 6 h.28 Frape et al. also demonstrated that subjects given a fatty breakfast followed by a fatty lunch produce larger postprandial glucose responses than individuals given a carbohydrate-rich breakfast followed by a fatty lunch.27, 28 Together, the above findings may explain why in our study increasing energy intake from carbohydrate in the morning at the expense of fat was associated with lower development of metabolic syndrome and abdominal obesity. The combined evidence also supports the concept that there are physiological windows of time when the human body favours the consumption of some nutrients but not others, with the ends of the day having the greatest influence.
In the present study, waist circumference was the component most influenced by the time of nutrient intake. These findings are particularly pertinent as elevated waist circumference is an important determinant of insulin resistance and cardiovascular disease risk. As a result, further research is required to validate our findings in other cohorts and in randomised controlled trials. Our study did not investigate the impact of environmental factors on nutrient distribution and did not examine whether differences in the nutrient composition of eating occasions between individuals with or without the metabolic syndrome may have arisen as a result of differences in the way the meals are eaten. The context of a meal is known to influence food choices and nutrient intake, with meals consumed alone or on the run being more likely to be of poorer nutrient content.48 This area warrants attention, particularly when considering differences in the social and environmental contexts of different meals through the day.
It is important to acknowledge some of the limitations of our analysis. First, the definition of meals in the diet diaries was subjective and not based on time. Second, we cannot exclude the possibility of residual confounding as we did not control for time of physical activity or variables such as sleep, which can potentially influence circadian rhythms and body composition.49 Low physical activity levels in the evening, in particular, have been hypothesised to account for the detrimental effects of evening/night eating,47 although currently no evidence exists to support or contradict this hypothesis. Underreporting is also a common issue with all dietary assessment methods. However, we were unaware of any data that can suggest that underreporting may influence the timing and nutrient composition of meals. Another limitation of the current analysis lies in the definition of the metabolic syndrome, which was defined using a modified ATPIII8 definition that included non-fasting glycosylated haemoglobin and lipids instead of fasting glucose and lipids. Finally, it is important to highlight that some of the observed protective effects of increasing carbohydrate at the expense of fat in the morning could be the result of substituting specific types of fat or carbohydrate, such as starch and sugars. Unfortunately, it was not possible to distinguish between the types of carbohydrate or fat in the present analysis. However, future studies should consider the types and sources of carbohydrate and fat.
Our study possesses several strengths. First, data were derived from a large prospective national birth cohort. Although sample loss is inevitable with long-term cohorts, the responding sample in NSHD remains in many respects representative of the national population of the same age.30 Losses due to death and emigration are similar to those in the national population of similar age,50 and trends in energy and nutrient intake are comparable to those reported in the national diet and nutrition survey.51 Rigorous dietary assessment methods were used to collect dietary data, and nutrient intake was estimated using food composition databases appropriate for the period of consumption. We used nutrient-density substitution models that take into account the complexity of the relationship between macronutrients and that an increase in one nutrient results in a simultaneous decrease in another macronutrient. Moreover, we have adjusted for total daily energy intake and energy intake at each eating occasion in our analyses, which indicates that the composition of eating occasions influences cardiometabolic parameters independently of daily energy intake or energy intake at the eating occasion.
In conclusion, time and nutrient composition of eating occasions predict the development of the metabolic syndrome and its individual components. These findings suggest that future dietary recommendations aimed at preventing or treating the metabolic syndrome should consider including advice on the time-of-day of nutrient intake alongside advice on overall nutrient quantity and quality. Attention should be drawn towards exploring other aspects of eating behaviour such as meal timing, frequency and regularity in attempt to unravel complex diet–disease interactions and improve our knowledge of the role of diet in maintaining optimum health.
References
Third Report of the National Cholesterol Education Program (NCEP). Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–3421.
Kahn R, Buse J, Ferrannini E, Stern M . The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005; 28: 2289–2304.
Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB . Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005; 112: 3066–3072.
Ford ES, Giles WH, Mokdad AH . Increasing prevalence of the metabolic syndrome among U.S. Adults. Diabetes Care 2004; 27: 2444–2449.
Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J . The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb 2005; 12: 295–300.
Ford ES . Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 2005; 28: 1769–1778.
Roche HM, Phillips C, Gibney MJ . The metabolic syndrome: the crossroads of diet and genetics. Proc Nutr Soc 2005; 64: 371–377.
Lutsey PL, Steffen LM, Stevens J . Dietary intake and the development of the metabolic syndrome: the atherosclerosis risk in communities study. Circulation 2008; 117: 754–761.
Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR . Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2009; 32: 688–694.
McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF . Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 2004; 27: 538–546.
Vessby B . Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr Opin Lipidol 2003; 14: 15–19.
Sonnenberg L, Pencina M, Kimokoti R, Quatromoni P, Nam BH, D'Agostino R et al. Dietary patterns and the metabolic syndrome in obese and non-obese Framingham women. Obes Res 2005; 13: 153–162.
Williams DE, Prevost AT, Whichelow MJ, Cox BD, Day NE, Wareham NJ . A cross-sectional study of dietary patterns with glucose intolerance and other features of the metabolic syndrome. Br J Nutr 2000; 83: 257–266.
Bray MS, Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O et al. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int J Obes (Lond) 34: 1589–1598.
Cho S, Dietrich M, Brown CJ, Clark CA, Block G . The effect of breakfast type on total daily energy intake and body mass index: results from the third National Health and Nutrition Examination Survey (NHANES III). J Am Coll Nutr 2003; 22: 296–302.
Deshmukh-Taskar PR, Nicklas TA, O'Neil CE, Keast DR, Radcliffe JD, Cho S . The relationship of breakfast skipping and type of breakfast consumption with nutrient intake and weight status in children and adolescents: the National Health and Nutrition Examination Survey 1999–2006. J Am Diet Assoc 2010; 110: 869–878.
Farshchi HR, Taylor MA, Macdonald IA . Beneficial metabolic effects of regular meal frequency on dietary thermogenesis, insulin sensitivity, and fasting lipid profiles in healthy obese women. Am J Clin Nutr 2005; 81: 16–24.
Farshchi HR, Taylor MA, Macdonald IA . Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr 2005; 81: 388–396.
Sierra-Johnson J, Unden AL, Linestrand M, Rosell M, Sjogren P, Kolak M et al. Eating meals irregularly: a novel environmental risk factor for the metabolic syndrome. Obesity (Silver Spring) 2008; 16: 1302–1307.
Whitton C, Nicholson SK, Roberts C, Prynne CJ, Pot GK, Olson A et al. National Diet and Nutrition Survey. UK food consumption and nutrient intakes from the first year of the rolling programme and comparisons with previous surveys. Br J Nutr 1–16.
De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L . Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol 2009; 38: 848–854.
Esquirol Y, Bongard V, Mabile L, Jonnier B, Soulat JM, Perret B . Shift work and metabolic syndrome: respective impacts of job strain, physical activity, and dietary rhythms. Chronobiol Int 2009; 26: 544–559.
Van Cauter E, Shapiro ET, Tillil H, Polonsky KS . Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol 1992; 262: E467–E475.
Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS . Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest 1991; 88: 934–942.
Morgan L, Hampton S, Gibbs M, Arendt J . Circadian aspects of postprandial metabolism. Chronobiol Int 2003; 20: 795–808.
Kario K . Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 56: 765–773.
Frape DL, Williams NR, Scriven AJ, Palmer CR, O'Sullivan K, Fletcher RJ . Diurnal trends in responses of blood plasma concentrations of glucose, insulin, and C-peptide following high- and low-fat meals and their relation to fat metabolism in healthy middle-aged volunteers. Br J Nutr 1997; 77: 523–535.
Frape DL, Williams NR, Rajput-Williams J, Maitland BW, Scriven AJ, Palmer CR et al. Effect of breakfast fat content on glucose tolerance and risk factors of atherosclerosis and thrombosis. Br J Nutr 1998; 80: 323–331.
Lund J, Arendt J, Hampton SM, English J, Morgan LM . Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol 2001; 171: 557–564.
Wadsworth ME, Butterworth SL, Hardy RJ, Kuh DJ, Richards M, Langenberg C et al. The life course prospective design: an example of benefits and problems associated with study longevity. Soc Sci Med 2003; 57: 2193–2205.
Holland B, Unwin ID, Buss DH . Cereals and cereal products: third supplement to McCance and Widdowson’s The Composition of Foods. Royal Society of Chemistry and Ministry of Agriculture, Fisheries and Food: Nottingham, 1988.
Holland B, Unwin ID, Buss DH . Milk and Milk Products: Fourth Supplement to McCance and Widdowson’s The Composition of Foods. Royal Society of Chemistry and Ministry of Agriculture Fisheries and Food: Cambridge, 1989.
Wadsworth M, Kuh D, Richards M, Hardy R . Cohort Profile: the 1946 National Birth Cohort (MRC National Survey of Health and Development). Int J Epidemiol 2006; 35: 49–54.
Willett WC, Howe GR, Kushi LH . Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997; 65: 1220S–1228S, discussion 1229S-1231S.
Skilton MR, Laville M, Cust AE, Moulin P, Bonnet F . The association between dietary macronutrient intake and the prevalence of the metabolic syndrome. Br J Nutr 2008; 100: 400–407.
Al-Naimi S, Hampton SM, Richard P, Tzung C, Morgan LM . Postprandial metabolic profiles following meals and snacks eaten during simulated night and day shift work. Chronobiol Int 2004; 21: 937–947.
Holmback U, Forslund A, Forslund J, Hambraeus L, Lennernas M, Lowden A et al. Metabolic responses to nocturnal eating in men are affected by sources of dietary energy. J Nutr 2002; 132: 1892–1899.
Holmback U, Forslund A, Lowden A, Forslund J, Akerstedt T, Lennernas M et al. Endocrine responses to nocturnal eating--possible implications for night work. Eur J Nutr 2003; 42: 75–83.
Lin YC, Hsiao TJ, Chen PC . Persistent rotating shift-work exposure accelerates development of metabolic syndrome among middle-aged female employees: a five-year follow-up. Chronobiol Int 2009; 26: 740–755.
Lin YC, Hsiao TJ, Chen PC . Shift work aggravates metabolic syndrome development among early-middle-aged males with elevated ALT. World J Gastroenterol 2009; 15: 5654–5661.
Sookoian S, Gemma C, Fernandez Gianotti T, Burgueno A, Alvarez A, Gonzalez CD et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med 2007; 261: 285–292.
Timlin MT, Pereira MA, Story M, Neumark-Sztainer D . Breakfast eating and weight change in a 5-year prospective analysis of adolescents: Project EAT (Eating Among Teens). Pediatrics 2008; 121: e638–e645.
Stanton JL, Keast DR . Serum cholesterol, fat intake, and breakfast consumption in the United States adult population. J Am Coll Nutr 1989; 8: 567–572.
Wolever TM, Bentum-Williams A, Jenkins DJ . Physiological modulation of plasma free fatty acid concentrations by diet. Metabolic implications in nondiabetic subjects. Diabetes Care 1995; 18: 962–970.
Lee A, Ader M, Bray GA, Bergman RN . Diurnal variation in glucose tolerance. Cyclic suppression of insulin action and insulin secretion in normal-weight, but not obese, subjects. Diabetes 1992; 41: 750–759.
Burdge GC, Jones AE, Frye SM, Goodson L, Wootton SA . Effect of meal sequence on postprandial lipid, glucose and insulin responses in young men. Eur J Clin Nutr 2003; 57: 1536–1544.
Kant AK, Schatzkin A, Ballard-Barbash R . Evening eating and subsequent long-term weight change in a national cohort. Int J Obes Relat Metab Disord 1997; 21: 407–412.
Larson NI, Nelson MC, Neumark-Sztainer D, Story M, Hannan PJ . Making time for meals: meal structure and associations with dietary intake in young adults. J Am Diet Assoc 2009; 109: 72–79.
Di Blasio A, Di Donato F, Mastrodicasa M, Fabrizio N, Di Renzo D, Napolitano G et al. Effects of the time of day of walking on dietary behaviour, body composition and aerobic fitness in post-menopausal women. J Sports Med Phys Fitness 2010; 50: 196–201.
Wadsworth ME, Mann SL, Rodgers B, Kuh DJ, Hilder WS, Yusuf EJ . Loss and representativeness in a 43 year follow up of a national birth cohort. J Epidemiol Community Health 1992; 46: 300–304.
Henderson L, Gregory J, Irving K . The National Diet and Nutrition Survey: adults aged 19 to 64 years Vol. 2, Energy, Protein, Carbohydrate, Fat and Alcohol Intake. The Stationary Office: London, 2003.
Acknowledgements
This work was supported by the UK National Prevention Research Initiative and the UK Medical Research Council (project number G701939).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Almoosawi, S., Prynne, C., Hardy, R. et al. Time-of-day and nutrient composition of eating occasions: prospective association with the metabolic syndrome in the 1946 British birth cohort. Int J Obes 37, 725–731 (2013). https://doi.org/10.1038/ijo.2012.103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2012.103
Keywords
This article is cited by
-
Meal-specific dietary patterns and biomarkers of insulin resistance in a sample of Iranian adults: a cross-sectional study
Scientific Reports (2023)
-
The association between meal-based diet quality index-international (DQI-I) with obesity in adults
BMC Nutrition (2022)
-
Meal timing across the day modulates daily energy intake in adult patients with type 2 diabetes
European Journal of Clinical Nutrition (2022)
-
Trajectories of energy intake distribution and subsequent risk of hyperglycemia among Chinese adults: findings from the China Health and Nutrition Survey (1997–2018)
European Journal of Nutrition (2022)
-
Chrononutrition in the management of diabetes
Nutrition & Diabetes (2020)