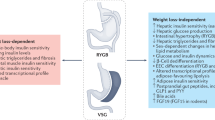
Abstract
Behavioral and pharmaceutical intervention to treat obesity and its comorbidities typically results in only a 5–10% weight loss. Thus, bariatric surgery is the most effective obesity treatment with some surgeries resulting in 30% sustained weight loss. Although this degree of weight loss has profound metabolic impact, these surgeries seem to have metabolic effects that are independent of weight loss. In support of this is the clinical literature showing rapid resolution of Type 2 diabetes mellitus (T2DM) that occurs before significant weight loss. To gain a complete understanding of the weight loss-independent effects of bariatric surgery, animal models have been developed. These are becoming more widely implemented and allow the use of pair-fed or weight-matched sham-operated controls in order to gain mechanistic insights into the mode of action of bariatric surgery. Increases in anorectic gut hormones, such as glucagon-like peptide-1 and peptide YY, or decreases in the orexigenic hormone ghrelin have been seen and are implicated as mediators of weight loss-independent actions of bariatric surgery. Changes in nutrient processing and sensing may also have a mechanistic role that is independent of, or that regulates, gut hormone responses to these surgeries. Ultimately, the hope is that understanding the mechanisms of bariatric surgeries will aid in the development of less invasive surgeries or pharmacological therapies that are more specifically, and perhaps individually, targeted at weight loss and/or resolution of T2DM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Makary MA, Clarke JM, Shore AD, Magnuson TH, Richards T, Bass EB et al. Medication utilization and annual health care costs in patients with type 2 diabetes mellitus before and after bariatric surgery. Arch Surg 2010; 145: 726–731.
Rubino F, Schauer PR, Kaplan LM, Cummings DE . Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med 2010; 61: 393–411.
Group TLAR, Wing R . Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the look AHEAD trial. Arch Intern Med 2010; 170: 1566–1575.
Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008; 299: 316–323.
Hofso D, Nordstrand N, Johnson LK, Karlsen TI, Hager H, Jenssen T et al. Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol 2010; 163: 735–745.
Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004; 351: 2683–2693.
Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008; 93: 2479–2485.
Shah SS, Todkar JS, Shah PS, Cummings DE . Diabetes remission and reduced cardiovascular risk after gastric bypass in Asian Indians with body mass index <35 kg/m2. Surg Obes Relat Dis 2010; 6: 332–338.
Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 2004; 240: 236–242.
Stylopoulos N, Hoppin AG, Kaplan LM . Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 2009; 17: 1839–1847.
Gatmaitan P, Huang H, Talarico J, Moustarah F, Kashyap S, Kirwan JP et al. Pancreatic islet isolation after gastric bypass in a rat model: technique and initial results for a promising research tool. Surg Obes Relat Dis 2010; 6: 532–537.
Bueter M, Lowenstein C, Olbers T, Wang M, Cluny NL, Bloom SR et al. Gastric bypass increases energy expenditure in rats. Gastroenterology 2010; 138: 1845–1853.
Rubino F, Marescaux J . Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004; 239: 1–11.
Koopmans HS, Sclafani A . Control of body weight by lower gut signals. Int J Obes 1981; 5: 491–495.
Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg 2006; 244: 741–749.
Rubino F, Zizzari P, Tomasetto C, Bluet-Pajot MT, Forgione A, Vix M et al. The role of the small bowel in the regulation of circulating ghrelin levels and food intake in the obese Zucker rat. Endocrinology 2005; 146: 1745–1751.
Cummings BP, Strader AD, Stanhope KL, Graham JL, Lee J, Raybould HE et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology 2010; 138: e2437–e2446, 2446.e1.
Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 2010; 52: 934–944.
Strader AD, Clausen TR, Goodin SZ, Wendt D . Ileal interposition improves glucose tolerance in low dose streptozotocin-treated diabetic and euglycemic rats. Obes Surg 2009; 19: 96–104.
Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Alessio DA, Seeley RJ . Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 2005; 288: E447–E453.
Abbatini F, Rizzello M, Casella G, Alessandri G, Capoccia D, Leonetti F et al. Long-term effects of laparoscopic sleeve gastrectomy, gastric bypass, and adjustable gastric banding on type 2 diabetes. Surg Endosci 2010; 24: 1005–1010.
de Bona Castelan J, Bettiol J, d’Acampora AJ, Castelan JV, de Souza JC, Bressiani V et al. Sleeve gastrectomy model in Wistar rats. Obes Surg 2007; 17: 957–961.
Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg 2009; 250: 234–241.
Rizzello M, Abbatini F, Casella G, Alessandri G, Fantini A, Leonetti F et al. Early postoperative insulin-resistance changes after sleeve gastrectomy. Obes Surg 2010; 20: 50–55.
Silecchia G, Boru C, Pecchia A, Rizzello M, Casella G, Leonetti F et al. Effectiveness of laparoscopic sleeve gastrectomy (first stage of biliopancreatic diversion with duodenal switch) on co-morbidities in super-obese high-risk patients. Obes Surg 2006; 16: 1138–1144.
Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 2010; 138: 2426–2436, 2436. e1–3.
Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK . Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 2008; 247: 401–407.
Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009; 33: 786–795.
le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 2007; 246: 780–785.
Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR . Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 2010; 151: 1588–1597.
Kindel TL, Yoder SM, Seeley RJ, D’Alessio DA, Tso P . Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg 2009; 13: 1762–1772.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K . Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999; 402: 656–660.
Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002; 346: 1623–1630.
Geloneze B, Tambascia MA, Pilla VF, Geloneze SR, Repetto EM, Pareja JC . Ghrelin: a gut-brain hormone: effect of gastric bypass surgery. Obes Surg 2003; 13: 17–22.
Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 2009; 297: R1273–R1282.
Li F, Zhang G, Liang J, Ding X, Cheng Z, Hu S . Sleeve gastrectomy provides a better control of diabetes by decreasing ghrelin in the diabetic Goto–Kakizaki rats. J Gastrointest Surg 2009; 13: 2302–2308.
Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K . Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2003; 88: 1594–1602.
Prudom C, Liu J, Patrie J, Gaylinn BD, Foster-Schubert KE, Cummings DE et al. Comparison of competitive radioimmunoassays and two-site sandwich assays for the measurement and interpretation of plasma ghrelin levels. J Clin Endocrinol Metab 2010; 95: 2351–2358.
Sugerman HJ, Kellum JM, Engle KM, Wolfe L, Starkey JV, Birkenhauer R et al. Gastric bypass for treating severe obesity. Am J Clin Nutr 1992; 55: 560S–566S.
Thirlby RC, Bahiraei F, Randall J, Drewnoski A . Effect of Roux-en-Y gastric bypass on satiety and food likes: the role of genetics. J Gastrointest Surg 2006; 10: 270–277.
Shin AC, Zheng H, Pistell PJ, Berthoud HR . Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 2011; 35: 642–651.
Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 2008; 8: 201–211.
Previs SF, Brunengraber DZ, Brunengraber H . Is there glucose production outside of the liver and kidney? Annu Rev Nutr 2009; 29: 43–57.
Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 1999; 284: 1365–1368.
Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H . Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 2009; 58: 1400–1407.
Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 2009; 10: 167–177.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D Sandoval has received grant support from NIH-NIDDK-K01 Career Development Award, NIH-NIDDK-R01 and Ethicon Endo-Surgery.
Rights and permissions
About this article
Cite this article
Sandoval, D. Bariatric surgeries: beyond restriction and malabsorption. Int J Obes 35 (Suppl 3), S45–S49 (2011). https://doi.org/10.1038/ijo.2011.148
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.148
Keywords
This article is cited by
-
Trends in Serum Vitamin D Levels within 12 Months after One Anastomosis Gastric Bypass (OAGB)
Obesity Surgery (2021)
-
Gut Microbiota in Patients with Morbid Obesity Before and After Bariatric Surgery: a Ten-Year Review Study (2009–2019)
Obesity Surgery (2021)
-
Gastric bypass surgery in a rat model alters the community structure and functional composition of the intestinal microbiota independently of weight loss
Microbiome (2020)
-
Sleep Apnea, Obesity, and Disturbed Glucose Homeostasis: Epidemiologic Evidence, Biologic Insights, and Therapeutic Strategies
Current Obesity Reports (2020)
-
Changes in Alcohol Use after Metabolic and Bariatric Surgery: Predictors and Mechanisms
Current Psychiatry Reports (2019)