Abstract
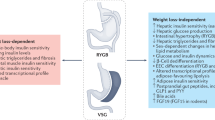
Gastric bypass surgery (GBP) results in important and sustained weight loss and remarkable improvement of Type 2 diabetes. The favorable change in the incretin gut hormones is thought to be responsible, in part, for diabetes remission after GBP, independent of weight loss. However, the relative role of the change in incretins and of weight loss is difficult to differentiate. After GBP, the plasma concentrations of the incretin hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide increase postprandially by three- to fivefold. The postprandial incretin effect on insulin secretion, blunted in diabetes, improves rapidly after the surgery. In addition to the change in incretins, the pattern of insulin secretion in response to oral glucose changes after GBP, with recovery of the early phase and significant decrease in postprandial glucose levels. These changes were not seen after an equivalent weight loss by diet. The improved insulin release and glucose tolerance after GBP were shown by others to be blocked by the administration of a GLP-1 antagonist, demonstrating that the favorable metabolic changes after GBP are, in part, GLP-1 dependent. The improved incretin levels and effect persist years after GBP, but their long-term effect on glucose metabolism, and on hypoglycemia post GBP are yet unknown. Understanding the mechanisms by which incretin release is exaggerated postprandially after GBP may help develop new less invasive treatment options for obesity and diabetes. Changes in rate of eating, gastric emptying, intestinal transit time, nutrient absorption and sensing, as well as bile acid metabolism, may all be implicated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004; 292: 1724–1737.
Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008; 299: 316–323.
Rossetti L, Giaccari A, DeFronzo RA . Glucose toxicity. Diabetes Care 1990; 13: 610–630.
Leahy JL, Bonner-Weir S, Weir GC . Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992; 15: 442–455.
Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007; 30: 1709–1716.
Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab 2008; 93: 2479–2485.
Kreymann B, Williams G, Ghatei MA, Bloom SR . Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 1987; 2: 1300–1304.
Holst JJ, Orskov C . Incretin hormones—an update. Scand J Clin Lab Invest Suppl 2001; 234: 75–85.
Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 2006; 290: E550–E559.
Ebert R, Creutzfeldt W . Gastrointestinal peptides and insulin secretion. Diabetes Metab Rev 1987; 3: 1–26.
Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab 1986; 63: 492–498.
Preitner F, Ibberson M, Franklin I, Binnert C, Pende M, Gjinovci A et al. Gluco-incretins control insulin secretion at multiple levels as revealed in mice lacking GLP-1 and GIP receptors. J Clin Invest 2004; 113: 635–645.
Flint A, Raben A, Ersboll AK, Holst JJ, Astrup A . The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord 2001; 25: 781–792.
Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol 1999; 276: R1541–R1544.
Naslund E, Bogefors J, Skogar S, Gryback P, Jacobsson H, Holst JJ et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol 1999; 277: R910–R916.
D’Alessio DA, Prigeon RL, Ensinck JW . Enteral enhancement of glucose disposition by both insulin-dependent and insulin-independent processes. A physiological role of glucagon-like peptide I. Diabetes 1995; 44: 1433–1437.
Nauck M, Stockmann F, Ebert R, Creutzfeldt W . Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29: 46–52.
Holst JJ . Glucagon-like peptide-1: from extract to agent. The Claude Bernard Lecture, 2005. Diabetologia 2006; 49: 253–260.
Sarson DL, Scopinaro N, Bloom SR . Gut hormone changes after jejunoileal (JIB) or biliopancreatic (BPB) bypass surgery for morbid obesity. Int J Obes 1981; 5: 471–480.
Naslund E, Gryback P, Hellstrom PM, Jacobsson H, Holst JJ, Theodorsson E et al. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord 1997; 21: 387–392.
Naslund E, Backman L, Holst JJ, Theodorsson E, Hellstrom PM . Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg 1998; 8: 253–260.
Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab 2006; 91: 1735–1740.
Sarson DL, Besterman HS, Bloom SR . Radioimmunoassay of gastric inhibitory polypeptide and its release in morbid obesity and after jejuno-ileal bypass [proceedings]. J Endocrinol 1979; 81: 155P–156P.
Lauritsen KB, Christensen KC, Stokholm KH . Gastric inhibitory polypeptide (GIP) release and incretin effect after oral glucose in obesity and after jejunoileal bypass. Scand J Gastroenterol 1980; 15: 489–495.
Halverson JD, Kramer J, Cave A, Permutt A, Santiago J . Altered glucose tolerance, insulin response, and insulin sensitivity after massive weight reduction subsequent to gastric bypass. Surgery 1982; 92: 235–240.
Kindel TL, Yoder SM, Seeley RJ, D’Alessio DA, Tso P . Duodenal-Jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg 2009; 13: 1762–1772.
Verdich C, Toubro S, Buemann B, Lysgard MJ, Juul HJ, Astrup A . The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord 2001; 25: 1206–1214.
Cohen RV, Schiavon CA, Pinheiro JS, Correa JL, Rubino F . Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: a report of 2 cases. Surg Obes Relat Dis 2007; 3: 195–197.
Naslund E, Backman L, Holst JJ, Theodorsson E, Hellstrom PM . Importance of small bowel peptides for the improved glucose metabolism 20 years after jejunoileal bypass for obesity. Obes Surg 1998; 8: 253–260.
Bose M, Teixeira J, Scherer PE, Pi-Sunyer FX, Bawa B, Laferrère B . Weight loss and incretin responsiveness improve glucose control independently after gastric bypass surgery. J Diabetes 2010; 2: 47–55.
Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV . Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005; 353: 249–254.
Kellogg TA, Bantle JP, Leslie DB, Redmond JB, Slusarek B, Swan T et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis 2008; 4: 492–499.
Farilla L, Bulotta A, Hirshberg B, Li CS, Khoury N, Noushmehr H et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 2003; 144: 5149–5158.
Cornu M, Yang JY, Jaccard E, Poussin C, Widmann C, Thorens B . Glucagon-like peptide-1 protects beta-cells against apoptosis by increasing the activity of an IGF-2/IGF-1 receptor autocrine loop. Diabetes 2009; 58: 1816–1825.
McLaughlin T, Peck M, Holst J, Deacon C . Reversible hyperinsulinemic hypoglycemia after gastric bypass: a consequence of altered nutrient delivery. J Clin Endocrinol Metab 2010; 95: 1851–1855.
Rubino F, Marescaux J . Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg 2004; 239: 1–11.
Pacheco D, de Luis DA, Romero A, Gonzalez SM, Conde R, Izaola O et al. The effects of duodenal-jejunal exclusion on hormonal regulation of glucose metabolism in Goto-Kakizaki rats. Am J Surg 2007; 194: 221–224.
Patriti A, Facchiano E, Annetti C, Aisa MC, Galli F, Fanelli C et al. Early improvement of glucose tolerance after ileal transposition in a non-obese type 2 diabetes rat model. Obes Surg 2005; 15: 1258–1264.
Strader AD, Vahl TP, Jandacek RJ, Woods SC, D’Alessio DA, Seeley RJ . Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab 2005; 288: E447–E453.
Horowitz M, Collins PJ, Harding PE, Shearman DJ . Gastric emptying after gastric bypass. Int J Obes 1986; 10: 117–121.
Gill RS, Birch DW, Shi X, Sharma AM, Karmali S . Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis 2010; 6: 707–713.
Olivan B, Teixeira J, Bose M, Bawa B, Chang T, Summe H et al. Effect of weight loss by diet or gastric bypass surgery on peptide YY3-36 levels. Ann Surg 2009; 249: 948–953.
Laferrère B, Swerdlow N, Bawa B, Arias S, Bose M, Olivan B et al. Rise of oxyntomodulin in response to oral glucose after gastric bypass surgery in patients with type 2 diabetes. J Clin Endocrinol Metab 2010; 95: 4072–4076.
Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002; 346: 1623–1630.
Bose M, Machineni S, Olivan B, Teixeira J, McGinty JJ, Bawa B et al. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity (Silver Spring) 2010; 18: 1085–1091.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
B Laferrère received funding from the American Diabetes Association CR-7-05 CR-18, NIH NIDDK R01-DK67561, GCRC 1 UL1 RR024156-02, ORC DK-26687, DERC DK-63068-05, Merck and Amylin Investigator Initiated Studies Program. B Laferrère also received lecture fees from Novo Nordisk.
Rights and permissions
About this article
Cite this article
Laferrère, B. Diabetes remission after bariatric surgery: is it just the incretins?. Int J Obes 35 (Suppl 3), S22–S25 (2011). https://doi.org/10.1038/ijo.2011.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2011.143
Keywords
This article is cited by
-
Single-Anastomosis Duodeno-ileal Bypass with Sleeve Gastrectomy as an Ultimate Option for Diabetics with Severe Obesity: A Scoping Review
Current Surgery Reports (2024)
-
Rise in Postprandial GLP-1 Levels After Roux-en-Y Gastric Bypass: Involvement of the Vagus Nerve–Spleen Anti-inflammatory Axis in Type 2 Diabetic Rats
Obesity Surgery (2022)
-
Efficacy of laparoscopic sleeve gastrectomy for patient with morbid obesity and type 1 diabetes mellitus: a case report
Surgical Case Reports (2021)
-
Impact of Duodeno-Jejunal Bypass Liner (EndoBarrierTM) Implantation on Insulin Sensitivity in Patients with Type 2 Diabetes Mellitus (T2DM): A Study Protocol for a Pilot Trial
Diabetes Therapy (2019)
-
Postprandial hypoglycaemia after Roux-en-Y gastric bypass in individuals with type 2 diabetes
Diabetologia (2019)