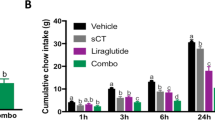
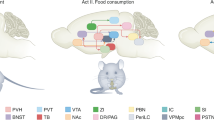
Abstract
Amylin is a pancreatic B-cell hormone that plays an important role in the control of nutrient fluxes because it reduces food intake, slows gastric emptying, and reduces postprandial glucagon secretion. These actions seem to depend on a direct effect on the area postrema (AP). Subsequent to area AP activation, the amylin signal is conveyed to the forebrain via distinct relay stations. Within the lateral hypothalamic area, amylin diminishes the expression of orexigenic neuropeptides. Recent studies suggest that amylin may also play a role as a long term, adiposity signal. Similar to leptin or insulin, an infusion of amylin into the brain resulted in lower body weight gain than in controls, irrespective of the starting body weight. Interestingly, preliminary data also suggest that rats fed an energy-dense diet develop resistance to central amylin. In addition to amylin's action to control meal termination and to act as a potential adiposity signal, amylin and its agonist salmon calcitonin have recently been shown to increase energy expenditure under certain conditions. In summary, amylin may be an interesting target as a body weight lowering drug. In fact, recent studies provide evidence that amylin, especially when combined with other anorectic hormones (for example, peptide YY and leptin) has beneficial long-term effects on body weight.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Young A, Denaro M . Roles of amylin in diabetes and in regulation of nutrient load. Nutrition 1998; 14: 524–527.
Hollander P, Maggs DG, Ruggles JA, Fineman M, Shen L, Kolterman OG et al. Effect of pramlintide on weight in overweight and obese insulin-treated type 2 diabetes patients. Obes Res 2004; 12: 661–668.
Chapman I, Parker B, Doran S, Feinle-Bisset C, Wishart J, Strobel S et al. Effect of pramlintide on satiety and food intake in obese subjects with type 2 diabetes. Diabetologia 2005; 48: 838–848.
Rushing PA, Hagan MM, Seeley RJ, Lutz TA, D’Alessio DA, Air EL et al. Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology 2001; 142: 5035.
Woods SC . Signals that influence food intake and body weight. Physiol Behav 2005; 86: 709–716.
Lutz TA . Pancreatic amylin as a centrally acting satiating hormone. Curr Drug Targets 2005; 6: 181–189.
Geary N . Endocrine controls of eating: CCK, leptin, and ghrelin. Physiol Behav 2004; 81: 719–733.
Reidelberger RD, Haver AC, Arnelo U, Smith DD, Schaffert CS, Permert J . Amylin receptor blockade stimulates food intake in rats. Am J Physiol Regul Integr Comp Physiol 2004; 287: R568–R574.
Mollet A, Gilg S, Riediger T, Lutz TA . Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiol Behav 2004; 81: 149–155.
Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E . The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes Relat Metab Disord 2001; 25: 1005–1011.
Lutz TA, Senn M, Althaus J, Del Prete E, Ehrensperger F, Scharrer E . Lesion of the area postrema/nucleus of the solitary tract (AP/NTS) attenuates the anorectic effects of amylin and calcitonin gene-related peptide (CGRP) in rats. Peptides 1998; 19: 309–317.
Morley JE, Flood JF, Horowitz M, Morley PMK, Walter MJ . Modulation of food intake by peripherally administered amylin. Am J Physiol 1994; 267: R178–R184.
Rowland NE, Richmond RM . Area postrema and the anorectic actions of dexfenfluramine and amylin. Brain Res 1999; 820: 86–91.
Riediger T, Zuend D, Becskei C, Lutz TA . The anorectic hormone amylin contributes to feeding-related changes of neuronal activity in key structures of the gut-brain axis. Am J Physiol Regul Integr Comp Physiol 2004; 286: R114–R122.
Michel S, Becskei C, Erguven E, Lutz TA, Riediger T . Diet-derived nutrients modulate the effects of amylin on c-Fos expression in the area postrema and on food intake. Neuroendocrinology 2007; 86: 124–135.
Becskei C, Grabler V, Edwards GL, Riediger T, Lutz TA . Lesion of the lateral parabrachial nucleus attenuates the anorectic effect of peripheral amylin and CCK. Brain Res 2007; 1162: 76–84.
Barth SW, Riediger T, Lutz TA, Rechkemmer G . Differential effects of amylin and salmon calcitonin on neuropeptide gene expression in the lateral hypothalamic area and the arcuate nucleus of the rat. Neurosci Lett 2003; 341: 131–134.
Bhavsar S, Watkins J, Young A . Synergy between amylin and cholecystokinin for inhibition of food intake in mice. Physiol Behav 1998; 64: 557–561.
Mollet A, Meier S, Grabler V, Gilg S, Scharrer E, Lutz TA . Endogenous amylin contributes to the anorectic effects of cholecystokinin and bombesin. Peptides 2003; 24: 91–98.
Eiden S, Daniel C, Steinbrueck A, Schmidt I, Simon EJ . Salmon calcitonin—a potent inhibitor of food intake in states of impaired leptin signalling in laboratory rodents. J Physiol 2002; 541: 1041–1048.
Osto M, Wielinga PY, Alder B, Walser N, Lutz TA . Modulation of the satiating effect of amylin by central ghrelin, leptin and insulin. Physiol Behav 2007; 91: 566–572.
Rushing PA, Lutz TA, Seeley RJ, Woods SC . Amylin and insulin interact to reduce food intake in rats. Horm Metab Res 2000; 32: 62–65.
Kobelt P, Goebel M, Stengel A, Schmidtmann M, van der Voort IR, Tebbe JJ et al. Bombesin, but not amylin, blocks the orexigenic effect of peripheral ghrelin. Am J Physiol Regul Integr Comp Physiol 2006; 291: R903–R913.
Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA 2008; 105: 7257–7262.
Chen HC, Roth JD, Schroeder BE, Weyer C . Role of islet-, gut-, and adipocyte-derived hormones in the central control of food intake and body weight: implications for an integrated neurohormonal approach to obesity pharmacotherapy. Curr Diabetes Rev 2008; 4: 79–91.
Mollet A, Meier S, Riediger T, Lutz TA . Histamine H1 receptors in the ventromedial hypothalamus mediate the anorectic action of the pancreatic hormone amylin. Peptides 2003; 24: 155–158.
Arnelo U, Permert J, Adrian TE, Larsson J, Westermark P, Reidelberger RD . Chronic infusion of IAPP causes anorexia in rats. Am J Physiol 1996; 271: R1654–R1659.
Isaksson B, Wang F, Permert J, Olsson M, Fruin B, Herrington MK et al. Chronically administered islet amyloid polypeptide in rats serves as an adiposity inhibitor and regulates energy homeostasis. Pancretology 2005; 5: 29–36.
Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC . Intraventricular insulin and the level of maintained body weight in rats. Behav Neurosci 1995; 109: 528–531.
Grabler V, Lutz TA . Chronic infusion of the amylin antagonist AC 187 increases feeding in Zucker fa/fa rats but not in lean controls. Physiol Behav 2004; 81: 481–488.
Wielinga PY, Alder B, Lutz TA . The acute effect of amylin and salmon calcitonin on energy expenditure. Physiol Behav 2007; 91: 212–217.
Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM . Antiobesity effects of the beta-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology 2006; 147: 5855–5864.
Acknowledgements
The financial support of the Swiss National Science Foundation and of the Zurich Center of Integrative Human Physiology are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lutz, T. Control of food intake and energy expenditure by amylin—therapeutic implications. Int J Obes 33 (Suppl 1), S24–S27 (2009). https://doi.org/10.1038/ijo.2009.13
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2009.13
Keywords
This article is cited by
-
Anti-obesity drug discovery: advances and challenges
Nature Reviews Drug Discovery (2022)
-
The Fight Against Obesity Escalates: New Drugs on the Horizon and Metabolic Implications
Current Obesity Reports (2020)
-
Future Pharmacotherapy for Obesity: New Anti-obesity Drugs on the Horizon
Current Obesity Reports (2018)
-
The gut sensor as regulator of body weight
Endocrine (2015)
-
Treatments for obesity: view from the Chair
International Journal of Obesity (2009)