Abstract
The aim of this systematic review and meta-analysis was to evaluate the effect of exercise training on parameters of the renin–angiotensin–aldosterone system (RAAS) in healthy adults, and to investigate the relation with training induced changes in blood pressure. A systematic search was conducted and we included randomized controlled trials lasting ⩾4 weeks investigating the effects of exercise on parameters of the RAAS in healthy adults (age ⩾18 years) and published in a peer-reviewed journal up to December 2013. Fixed effects models were used and data are reported as weighted means and 95% confidence limits (CL). Eleven randomized controlled trials with a total of 375 individuals were included. Plasma renin activity was reduced after exercise training (n= 7 trials, standardized mean difference −0.25 (95% CL −0.5 to −0.001), P=0.049), whereas no effect was observed on serum aldosterone ((n= 3 trials; standardized mean difference −0.79 (−1.97 to +0.39)) or angiotensin II (n=3 trials; standardized mean difference −0.16 (−0.61 to +0.30). Significant reductions in systolic blood pressure −5.65 mm Hg (−8.12 to −3.17) and diastolic blood pressure −3.64 mm Hg (−5.4 to −1.91) following exercise training were observed. No relation was found between net changes in plasma renin activity and net changes in blood pressure (P>0.05). To conclude, although we observed a significant reduction in plasma renin activity following exercise training this was not related to the observed blood pressure reduction. Given the small number of studies and small sample sizes, larger well-controlled randomized studies are required to confirm our results and to investigate the potential role of the RAAS in the observed improvements in blood pressure following exercise training.
Similar content being viewed by others
Introduction
High blood pressure (BP) is an important risk factor for cardiovascular diseases; about 54% of stroke, 47% of ischaemic heart disease, and 25% of other cardiovascular diseases worldwide are attributable to high BP.1 It is widely accepted that regular exercise reduces office BP2, 3 and ambulatory BP values,3 particularly in hypertensive individuals,2, 3 and is recommended in the prevention and treatment of hypertension to assist in BP controlling.4, 5 However, the BP lowering mechanisms of exercise training remain largely elusive.2 Increased activation of the renin–angiotensin aldosterone system (RAAS) is associated with the development of hypertension. To date, most of the known biological actions of the RAAS appear to be mediated by angiotensin II (AngII) which is generated through an enzymatic cascade in which angiotensinogen is cleaved by renin to form angiotensin I, which, in turn, is converted to AngII by angiotensin-converting enzyme.6 AngII has direct action on renal tubular sodium retention and stimulates the secretion of aldosterone in the adrenal glands, which in turn determinates salt and water reuptake by the kidneys.6 As such the RAAS is one of the most powerful regulators of arterial BP as it influences sodium balance, extracellular fluid volume and renal and systemic vascular resistance.6 Previous trials that have investigated the effect of exercise training on parameters of the RAAS system in healthy individuals included small sample sizes and yielded variable results, resulting in inconclusive findings on the effect of exercise on the RAAS system and as a BP lowering mechanism following exercise.7, 8, 9, 10, 11, 12, 13, 14, 15, 16 By contrast, a decade ago, a meta-analysis by our group suggested that the mechanisms for the BP lowering effect of exercise training included a reduction in systemic vascular resistance in which the renin–angiotensin system might have a role.2 That is, following exercise training we observed a significant reduction both in BP and plasma renin activity (PRA). However, this was based on eight small trials and no metaregression analysis was performed at that time. Further, PRA was the only parameter related to the RAAS which was assessed. Since then, the number of eligible trials has increased, which should allow a more precise estimate of the overall effect of exercise training on RAAS. Therefore, the aim of this study was to perform a systematic review and meta-analysis (1) to investigate the effect of exercise training on different markers of the RAAS and (2) to determine the relation between exercise-induced changes in BP and changes in parameters of RAAS.
Methods
Search strategy and study selection
We performed a systematic literature search in the electronic Pubmed database from its inception to December 2013. Search terms included a mix of Medical Subject Headings (MeSH-terms) and free- text words for key concepts related to exercise training and the RAAS in clinical trials. These were combined with a sensitive search strategy to identify ‘randomized controlled trials’ performed in ‘humans’. The search criteria for the database search (PubMed) are shown in Supplementary File 1. In addition, we reviewed the reference lists of the original articles and reviews within this field of study to identify other possible eligible trials. The inclusion criteria for this meta-analysis were as follows: (1) randomized, controlled trials involving exercise training (aerobic, resistance or combined) of at least 4 weeks duration as the sole intervention; (2) participants were healthy adults (age ⩾18 years) with an optimal BP, prehypertension or hypertension and with no other concomitant disease; (3) data on PRA, plasma aldosterone and/or AngII were available; and finally, (4) the article was published in a peer-reviewed journal up to December 2013. Exclusion criteria included any studies not meeting all criteria above.
Studies included in the review
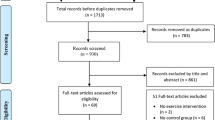
Our initial search identified 1246 papers, and through manual searching we identified a further six manuscripts.10, 11, 12, 13, 17 Out of 1252 trials, 1198 were excluded after reading titles or abstracts; of the remaining 54 we excluded an additional 43 studies for various reasons (including two studies which did not provide data that could be included in our analysis),18, 19 leaving 11 studies for the final analysis (Figure 1).
Data extraction and quality assessment
Data on study source, study design, study quality, sample size, characteristics of participants and exercise programs and outcomes of the interventions were extracted independently by two authors (KG and VAC) using a specific developed electronic data extraction sheet. Using Cohen’s kappa statistic, the overall agreement rate prior to correcting discrepant items was 0.92. Subsequently, disagreements were resolved by discussion. A priori, the primary outcomes were changes in measures of the RAAS system (PRA, serum aldosterone, AngII).
Study quality was assessed using an adapted PEDro-scale.20 That is, we regarded the quality criteria ‘blinding of participants’ and ‘blinding of therapists’ as not applicable in the included exercise studies, and omitted both criteria. All questions were bipolar (yes or no). The minimum score was 0 and the maximum was 8, with a higher number reflecting a better study quality. The PEDro-scale has been reported to be valid21 and reliable.22 All assessments were conducted in duplicate, independent of each other. Disagreements were resolved through consensus. Trials were not excluded on the basis of quality.
Statistical analyses
Descriptive analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC, USA), reliability statistics using Excel 2010 and meta-analytic statistics were performed by means of Comprehensive Meta-Analysis software (version 2.2, Biostat, Englewood, NJ). Data are reported as mean±s.d., median (range) or weighted mean and 95% confidence intervals (CI). Effect sizes for each study group from each parallel trial were calculated by subtracting the pre-exercise value from the post-exercise value (post–pre) for both the exercise (Δ1) and control groups (Δ2). The net treatment effect was then obtained as Δ1−Δ2. For cross-over trials,12 the net treatment effect was calculated as the difference at the end of the exercise and sedentary periods, respectively. Each effect size was weighted by the inverse of its variance. For studies with multi-intervention groups,9, 12 data from the exercise group that were reported to have generated the largest effect on the studied parameter in their respective trials were included. As the RAAS-parameters were assessed and reported by different methods/units, the mean differences were standardized by dividing them by the within-group s.d. The standardized mean difference (SMD) values in each trial were pooled with a fixed effects model. According to Cohen guidelines, SMD values of 0.2, 0.5 and 0.8 represent small, medium and large effect sizes, respectively. We statistically assessed inconsistency using I2. The I2 statistic between 25 and 50% represents small amounts of inconsistency, whereas between 50 and 75%, and >75% represents medium to large amounts of heterogeneity. As no significant heterogeneity was found for any of the outcome measures, the results are reported on the basis of fixed-effects models, except for aldosterone for which we used a random-effects model.
Univariate weighted meta-regressional analyses were conducted to determine the association among effect size changes in PRA and participants age, baseline resting BP, net changes in BP, net changes in weight and the duration of the intervention.
To evaluate the included studies for publication bias, we visually explored funnel plots for asymmetry. In addition, Begg's test23 and Egger's test24 were performed. Two-sided P<0.05 was considered statistically significant.
Results
Trial characteristics
Figure 1 shows the total number of studies that were identified and excluded at different stages of the selection process. Ultimately, we identified 11 trials7, 8, 9, 10, 11, 12, 13, 14, 15, 16 that fulfilled the inclusion criteria. Detailed characteristics of each study included in the meta-analysis are provided in Table 1. The trials were conducted between 1986 and 2011. Subjects in five studies came from the USA, one included subjects from South America, three included subjects from Asia and two studies selected subjects from Australia. The sample size of the trials at baseline ranged from 12 to 90 participants (median 27), totaling 375 randomized participants. The median age of the participants was 52.5 years (range 22–68 years). One trial included only women,16 whereas the other 10 trials included both men and women. In the final analysis 112 participants were men, 181 female and 20 unknown gender.10 Resting BP was reported in 10 trials and averaged 136 mm Hg (range 105–155 mm Hg)/78 mm Hg (range 51–99 mm Hg) at baseline. In relation to medication, seven trials reported that none of the participants used antihypertensive medications,7, 9, 12, 13, 14, 16 in two trial patients were withdrawn from antihypertensive medication before the start of the trial10, 15 whereas two other trials did not report on medication.8, 11 Participants in eight trials were considered sedentary at baseline, whereas the remaining three trials did not report on levels of habitual physical activity of their participants before engaging in the study.10, 13, 14 The duration of the interventions varied from 4 to 37 weeks (median 12) and frequency of exercise ranged from 3 to 6 days per week (median 3). Mode of exercise was isolated aerobic endurance training in eight trials,10, 11, 12, 13, 14, 15, 16 dynamic resistance training was performed in one trial7 and another two trials used a combination of endurance and dynamic resistance training.8, 9 Seven trials had participants perform only supervised training,7, 10, 12, 13, 14, 15 whereas one trial reported that participants performed one out of four session on their own9 and participants of another trial underwent two supervised and additional one to three home-based sessions.16 Two other trials did not specifically report whether exercise was supervised or home based.8, 11 None of the studies specifically reported whether the physical activity habits of the participants in the exercise and control groups changed outside the intervention itself, but all investigators instructed their participants (exercise and control) not to modify their usual lifestyle, including nutrition and physical activity. Further, three trials7, 9, 15 reported that they tried to control for placebo effect by offering a placebo treatment including, supervised stretching sessions7, 9 or isometric calisthenics.15
Study quality
Table 2 shows the results of the study quality using the adapted PEDro-score. Overall, the study quality of the included studies was high with a median PEDro-score of 6, (range 5 to 7). However, none of the included studies reported using concealed allocation and intention-to-treat analysis was only used in 45% of the studies.
Effect of exercise on the renin–angiotensin–aldosterone system and blood pressure
As shown in Figures 2,3,4, exercise significantly lowered PRA (−0.254 (−0.50 to−0.001), P=0.049; n=7 studies; I2=8; p for heterogeneity=0.37), but did not significantly affect levels of AngII (−0.16(−0.61 to +0.30); n=3 studies; I2=0; P for heterogeneity=0.68)) or serum aldosterone (−0.79 (−1.97 to +0.39); n=3 studies; I2=86.1; P for heterogeneity=0.001). Heterogeneity and inconsistency were low for all parameters, except serum aldosterone. Table 3 shows the results of exercise training on BP for all the included studies and for studies reporting on each of the RAAS-components. Overall, we observed a significant reduction in SBP −5.65 mmHg (−8.12 to −3.17) and DBP −3.64 mmHg (−5.4 to −1.91) following exercise training. Finally, weighted single metaregression analysis showed no significant associations between baseline BP, age, duration of the intervention, net changes in resting BP, net changes in weight and changes in renin activity (P>0.5 for all; data not shown).
Publication bias
Funnel plots for AngII, PRA and aldosterone did not show any significant publication bias for all of the outcomes, meaning that there was no asymmetric relationship between treatment effects and study size (Supplementary Figures S1–S3).
Discussion
The present meta-analysis of 11 RCT’s identified a significant reduction in PRA following exercise training in healthy adults which went along but was not associated with reductions in SBP and DBP. Further, no effect of exercise training was observed on levels of AngII and aldosterone.
Plasma renin activity can be affected by a number of lifestyle factors, among which are acute and chronic physical exercises. Cross-sectional studies have shown that PRA is lower in athletes than in untrained subjects.25, 26 Further, an inverse relationship has been demonstrated between PRA and levels of physical activity in healthy mildly hypertensive individuals27 and PRA and levels of physical fitness.28 However, results from longitudinal prospective trials12 and individual randomized controlled trials7, 8, 9, 10, 11, 12, 13, 14, 15, 16 are less conclusive with some studies reporting a decrease12 in PRA following chronic exercise training whereas others did not.7, 8, 10, 11, 12, 13, 14, 15, 16, 29 The present observation of reduced PRA following exercise training is in line with the results of our previous meta-analysis2 on the effect of exercise training on BP where we showed 20% lower values of PRA following exercise training in healthy adults with normal BP, prehypertension and hypertension. Further we also observed a significant reduction in BP following these exercise programs. However, no relation between changes in BP and changes in PRA could be established with metaregression analysis. The latter does however not exclude a potential role for the RAAS in the BP response post training. A combination of local endothelial derived factors, sympathetic nervous system, changes in endocrine secretions and last but not least changes in renal hemodynamics are responsible for the control of BP. Training induced changes in BP appear to be the result of a combination of these different mechanisms,30 although many questions still remain about causal relations and might be different in different populations and following different exercise program characteristics. With regard to the latter we were not able to demonstrate a relation between training duration and changes in PRA. Further, as all included studies involving PRA had their participants exercising at moderate intensity (that is, between 50 and 70% VO2max) metaregression analysis could not be performed. On the other hand, Matsusaki et al. showed earlier that 10 weeks of exercise training at 50% VO2max decreased PRA whereas exercise at an intensity larger than 75% VO2max increased PRA in hypertensive patients.31 However, future randomized controlled studies are needed to confirm their findings. Because all included PRA studies involved aerobic type of exercises, it also remains to be elucidated whether resistance training will induce similar effects on PRA. Finally, Nelson et al. and Jennings et al.12, 32 did not find a significant difference in PRA following either three times per week or seven times per week cycling at moderate intensity. This is in line with the effect of training frequency on BP where we showed earlier that training frequency did not affect BP responses.33
The RAAS is considered as an endocrine system with kidney-derived renin regulating the production of AngII.34 It is of note that renin by itself does not really affect the resting BP. Instead, it floats around and converts inactive forms of angiotensin into angiotensin I. Angiotensin I is able to alter BP to some degree but most angiotensin I is converted to AngII, a much more powerful hormone that induces large changes in BP as it acts on adrenals to stimulate the production of aldosterone and on cardiovascular and other tissues to regulate BP.6, 34 Whereas we observed a significant decrease in PRA after training, this could not be established for AngII or aldosterone. The reason for this is not clear. However, the fact that only three small studies included in this review reported on these parameters most likely has resulted in a lack of power to detect changes following exercise training. Further two of the three studies reporting on AngII adapted a dynamic resistance training protocol which has been shown to result in smaller reductions in BP33 compared to endurance training, as was also observed here. So it might be that the exercise programs investigating the effect on AngII and aldosterone were not the most appropriate to induce significant changes in these parameters. Further, Kohno et al.35 demonstrated that individuals with the highest basal renin levels showed larger reductions in BP and PRA post-exercise. They concluded that exercise training acts on BP by suppressing activity of RAAS only in individuals in which the system is more activated.35 The absence of a change in both AngII and aldosterone might suggest that there is no hyperactivity of the system. Further, reductions or increases in the concentrations of renin do not necessarily entail changes in other components of the RAAS. Earlier, Vanhees et al.36 and Geyssant A et al.37 found no effect on plasma AngI, plasma AngII or plasma aldosterone concentrations in coronary artery disease patients36 and healthy controls37 following exercise training despite a significant reduction in renin. Also, older cross-sectional studies found normal plasma AngII and aldosterone concentrations in spite of a reduced PRA in well-trained athletes compared to healthy controls.38 Contrary, Braith et al. 39 reported significant reductions in AngII and aldosterone following 16 weeks of endurance training in 19 heart failure patients; unfortunately no data were provided on PRA. However, it is well accepted that in this particular patient population progression of chronic heart failure is associated with activation of the RAAS that might result in a different response to exercise training compared with healthy individuals. Finally, in recent years it has gradually become clear that the physiology of the RAAS is by far more complex and multilayered than once thought.34, 40 There are different pathways that make up the system (that is, AngI, AngII, AngIII, AngIV, Ang1-7 and Ang1-9) in combination with different receptors that trigger divers responses.34, 40 Further studies are needed to investigate the possible effects of exercise training on alternative pathways of the RAAS.
However, it is known that some RAAS polymorphisms are associated with the BP response to exercise, or at least acute exercise.41, 42 Therefore, a role for the RAAS cannot be ruled out, at least in some genetically predisposed individuals. For example, individuals with DD genotype showed higher ACE concentration compare to heterozygotes individuals.43 Thus, higher ACE levels promoted increase on AngII formation and parallel lower formation or degradation of bradykinin and nitric oxide, favoring BP increase. Further, in response to exercise training, DI/II individuals demonstrated greater reductions in SBP compared with DD individuals.44
Finally, although PRA levels are usually higher in overweight individuals,29, 45 no relation between changes in weight and changes in PRA could be established here. Future studies should also investigate whether weight loss is a mechanisms of training induced changes in parameters of the RAAS as has been observed earlier.46
Finally, the results of this meta-analysis should be seen within the context of its limitations. First of all, the number of randomized controlled trials that have investigated the effect of exercise on parameters of the RAAS is limited and the sample sizes were small. Further, it should be acknowledged that AngI and AngII are structurally similar and the concentrations of AngII in the plasma are low making it difficult to obtain pure methods.47 And although Hikada et al.48 showed that their developed radioimmunoassay method is highly sensitive to detect small concentrations of AngII it might still be that the method used in two of our trials is not sensitive enough to detect even smaller changes in AngII following exercise. In addition, it is well-known that salt intake is one of the most important determinants of PRA levels. However, as we only included randomized controlled trials it seems reasonable to assume that salt intake at baseline and/or changes in salt intake would be the same in the exercise and control group throughout the trial. The latter was confirmed by five8, 10, 13, 14, 32 of the included studies reporting similar levels of urinary sodium excretion in both groups suggesting that salt intake remained unchanged.49 Hence, we believe that our results with regard to PRA can be explained by exercise and are not due to changes in salt intake.
In conclusion, we observed a significant reduction in BP and PRA following exercise training, but this could not be established for AngII and aldosterone. Further, metaregression revealed no relation between exercise-induced changes in BP and exercise-induced changes in BP. Larger and more rigorous studies are definitively needed to elucidate the role of the RAAS in exercise-induced changes in BP. These future RCTs should ensure sound methodology and reporting, mainly adequate sample size, adequate randomization, allocation concealment, intention to treat analysis. They should evaluate the effect of exercise on multiple components of the RAAS and taking into account different exercise program variables and patient characteristics.
References
Lawes CM, Vander Hoorn S, Rodgers A . Global burden of blood-pressure-related disease, 2001. Lancet 2008; 371: 1513–1518.
Cornelissen VA, Fagard RH . Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension 2005; 46: 667–675.
Cornelissen VA, Buys R, Smart NA . Endurance exercise beneficially affects ambulatory blood pressure: A systematic review and meta-analysis. J Hypertens 2013; 31: 639–648.
Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA . American college of sports medicine position stand. Exercise and hypertension. Med Sci Sports Exerc 2004; 36: 533–553.
Vanhees L, Geladas N, Hansen D, Kouidi E, Niebauer J, Reiner Z, Cornelissen V, Adamopoulos S, Prescott E, Borjesson M, Bjarnason-Wehrens B, Bjornstad HH, Cohen-Solal A, Conraads V, Corrado D, De Sutter J, Doherty P, Doyle F, Dugmore D, Ellingsen O, Fagard R, Giada F, Gielen S, Hager A, Halle M, Heidbuchel H, Jegier A, Mazic S, McGee H, Mellwig KP, Mendes M, Mezzani A, Pattyn N, Pelliccia A, Piepoli M, Rauch B, Schmidt-Trucksass A, Takken T, van Buuren F, Vanuzzo D . Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular risk factors: Recommendations from the eacpr. Part ii. Eur J Prev Cardiol 2012; 19: 1005–1033.
MLN J . The renin-angiotensin-aldosterone sytem BELGB GL Hypertension: Principles and Practice. Taylor & Francis group: Boca Raton. 2005, 143–156.
Anton MM, Cortez-Cooper MY, DeVan AE, Neidre DB, Cook JN, Tanaka H . Resistance training increases basal limb blood flow and vascular conductance in aging humans. J Appl Physiol (1985) 2006; 101: 1351–1355.
Carroll JF, Convertino VA, Wood CE, Graves JE, Lowenthal DT, Pollock ML . Effect of training on blood volume and plasma hormone concentrations in the elderly. Med Sci Sports Exerc 1995; 27: 79–84.
Cortez-Cooper MY, Anton MM, Devan AE, Neidre DB, Cook JN, Tanaka H . The effects of strength training on central arterial compliance in middle-aged and older adults. Eur J Cardiovasc Prev Rehabil 2008; 15: 149–155.
Hagberg JM, Montain SJ, Martin WH 3rd, Ehsani AA . Effect of exercise training in 60- to 69-year-old persons with essential hypertension. Am J Cardiol 1989; 64: 348–353.
Higashi Y, Sasaki S, Sasaki N, Nakagawa K, Ueda T, Yoshimizu A, Kurisu S, Matsuura H, Kajiyama G, Oshima T . Daily aerobic exercise improves reactive hyperemia in patients with essential hypertension. Hypertension 1999; 33: 591–597.
Jennings G, Nelson L, Nestel P, Esler M, Korner P, Burton D, Bazelmans J . The effects of changes in physical activity on major cardiovascular risk factors, hemodynamics, sympathetic function, and glucose utilization in man: A controlled study of four levels of activity. Circulation 1986; 73: 30–40.
Sakai T, Ideishi M, Miura S, Maeda H, Tashiro E, Koga M, Kinoshita A, Sasaguri M, Tanaka H, Shindo M, Arakawa K . Mild exercise activates renal dopamine system in mild hypertensives. J Hum Hypertens 1998; 12: 355–362.
Urata H, Tanabe Y, Kiyonaga A, Ikeda M, Tanaka H, Shindo M, Arakawa K . Antihypertensive and volume-depleting effects of mild exercise on essential hypertension. Hypertension 1987; 9: 245–252.
Waib PH, Goncalves MI, Barrile SR . Improvements in insulin sensitivity and muscle blood flow in aerobic-trained overweight-obese hypertensive patients are not associated with ambulatory blood pressure. J Clin Hypertens (Greenwich) 2011; 13: 89–96.
Yoshizawa M, Maeda S, Miyaki A, Misono M, Choi Y, Shimojo N, Ajisaka R, Tanaka H . Additive beneficial effects of lactotripeptides and aerobic exercise on arterial compliance in postmenopausal women. Am J Physiol Heart Circ Physiol 2009; 297: H1899–H1903.
Cornelissen VA, Arnout J, Holvoet P, Fagard RH . Influence of exercise at lower and higher intensity on blood pressure and cardiovascular risk factors at older age. J Hypertens 2009; 27: 753–762.
Hespel P, Lijnen P, Van Hoof R, Fagard R, Goossens W, Lissens W, Moerman E, Amery A . Effects of physical endurance training on the plasma renin-angiotensin-aldosterone system in normal man. J Endocrinol 1988; 116: 443–449.
Warburton DE, Haykowsky MJ, Quinney HA, Blackmore D, Teo KK, Taylor DA, McGavock J, Humen DP . Blood volume expansion and cardiorespiratory function: Effects of training modality. Med Sci Sports Exerc. 2004; 36: 991–1000.
Centre for Evidence-Based PhysiotherapyPhysiotherapy TCfE-B. Physiotherapy evidence database (pedro) 2014, http://www.pedro.org.au/.
de Morton NA . The pedro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust J Physiother 2009; 55: 129–133.
Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M . Reliability of the pedro scale for rating quality of randomized controlled trials. Phys Ther 2003; 83: 713–721.
Begg CB . A comparison of methods to detect publication bias in meta-analysis by p. Macaskill, s. D. Walter and l. Irwig, Statistics in Medicine, 2001; 20:641-654. Stat Med 2002; 21: 1803 author reply 1804.
Egger M, Davey Smith G, Schneider M, Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634.
Fagard R, Grauwels R, Groeseneken D, Lijnen P, Staessen J, Vanhees L, Amery A . Plasma levels of renin, angiotensin ii, and 6-ketoprostaglandin f1 alpha in endurance athletes. J Appl Physiol (1985) 1985; 59: 947–952.
Lijnen P, Hespel P, Van Oppens S, Fiocchi R, Goossens W, Vanden Eynde E, Amery A . Erythrocyte 2,3-diphosphoglycerate and serum enzyme concentrations in trained and sedentary men. Med Sci Sports Exerc 1986; 18: 174–179.
Palatini P, Canali C, Graniero GR, Rossi G, de Toni R, Santonastaso M, dal Follo M, Zanata G, Ferrarese E, Mormino P, Pessina AC . Relationship of plasma renin activity with caffeine intake and physical training in mild hypertensive men. Harvest Study Group. Eur J Epidemiol 1996; 12: 485–491.
M'Buyamba-Kabangu JR, Fagard R, Lijnen P, Amery A . Relationship between plasma renin activity and physical fitness in normal subjects. Eur J Appl Physiol Occup Physiol 1985; 53: 304–307.
Cooper R, McFarlane-Anderson N, Bennett FI, Wilks R, Puras A, Tewksbury D, Ward R, Forrester T . Ace, angiotensinogen and obesity: A potential pathway leading to hypertension. J Hum Hypertens 1997; 11: 107–111.
Hamer M . The anti-hypertensive effects of exercise: Integrating acute and chronic mechanisms. Sports Med 2006; 36: 109–116.
Matsusaki M, Ikeda M, Tashiro E, Koga M, Miura S, Ideishi M, Tanaka H, Shindo M, Arakawa K . Influence of workload on the antihypertensive effect of exercise. Clin Exp Pharmacol Physiol 1992; 19: 471–479.
Nelson L, Jennings GL, Esler MD, Korner PI . Effect of changing levels of physical activity on blood-pressure and haemodynamics in essential hypertension. Lancet 1986; 2: 473–476.
Cornelissen VA, Smart NA . Exercise training for blood pressure: A systematic review and meta-analysis. J Am Heart Assoc 2013; 2: e004473.
Aroor AR, Demarco VG, Jia G, Sun Z, Nistala R, Meininger GA, Sowers JR . The role of tissue renin-angiotensin-aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front Endocrinol (Lausanne) 2013; 4: 161.
Kohno K, Matsuoka H, Takenaka K, Miyake Y, Nomura G, Imaizumi T . Renal depressor mechanisms of physical training in patients with essential hypertension. Am J Hypertens 1997; 10: 859–868.
Vanhees L, Fagard R, Lijnen P, Moerman E, De Geest H, Amery A . Influence of physical training on blood pressure, plasma renin, angiotensin and catecholamines in patients with ischaemic heart disease. Eur J Appl Physiol Occup Physiol 1984; 53: 219–224.
Geyssant A, Geelen G, Denis C, Allevard AM, Vincent M, Jarsaillon E, Bizollon CA, Lacour JR, Gharib C . Plasma vasopressin, renin activity, and aldosterone: Effect of exercise and training. Eur J Appl Physiol Occup Physiol 1981; 46: 21–30.
Melin B, Eclache JP, Geelen G, Annat G, Allevard AM, Jarsaillon E, Zebidi A, Legros JJ, Gharib C . Plasma avp, neurophysin, renin activity, and aldosterone during submaximal exercise performed until exhaustion in trained and untrained men. Eur J Appl Physiol Occup Physiol 1980; 44: 141–151.
Braith RW, Welsch MA, Feigenbaum MS, Kluess HA, Pepine CJ . Neuroendocrine activation in heart failure is modified by endurance exercise training. J Am Coll Cardiol 1999; 34: 1170–1175.
Fyhrquist F, Saijonmaa O . Renin-angiotensin system revisited. J Intern Med 2008; 264: 224–236.
Blanchard BE, Tsongalis GJ, Guidry MA, LaBelle LA, Poulin M, Taylor AL, Maresh CM, Devaney J, Thompson PD, Pescatello LS . Raas polymorphisms alter the acute blood pressure response to aerobic exercise among men with hypertension. Eur J Appl Physiol 2006; 97: 26–33.
Pescatello LS, Turner D, Rodriguez N, Blanchard BE, Tsongalis GJ, Maresh CM, Duffy V, Thompson PD . Dietary calcium intake and renin angiotensin system polymorphisms alter the blood pressure response to aerobic exercise: A randomized control design. Nutr Metab (Lond) 2007; 4: 1.
Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F . An insertion/deletion polymorphism in the angiotensin i-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 1990; 86: 1343–1346.
Santana HA, Moreira SR, Neto WB, Silva CB, Sales MM, Oliveira VN, Asano RY, Espindola FS, Nobrega OT, Campbell CS, Simoes HG . The higher exercise intensity and the presence of allele i of ace gene elicit a higher post-exercise blood pressure reduction and nitric oxide release in elderly women: An experimental study. BMC Cardiovasc Disord 2011; 11: 71.
Egan BM, Stepniakowski K, Goodfriend TL . Renin and aldosterone are higher and the hyperinsulinemic effect of salt restriction greater in subjects with risk factors clustering. Am J Hypertens 1994; 7: 886–893.
Dall'Asta C, Vedani P, Manunta P, Pizzocri P, Marchi M, Paganelli M, Folli F, Pontiroli AE . Effect of weight loss through laparoscopic gastric banding on blood pressure, plasma renin activity and aldosterone levels in morbid obesity. Nutr Metab Cardiovasc Dis 2009; 19: 110–114.
Simon D, Romestand B, Huang H, Badouaille G, Fehrentz JA, Pau B, Marchand J, Corvol P . Direct, simplified, and sensitive assay of angiotensin ii in plasma extracts performed with a high-affinity monoclonal antibody. Clin Chem 1992; 38: 1963–1967.
Hidaka H, Sawada S, Sato R, Oka H . An improved method for measuring angiotensin i converting enzyme activity using a highly sensitive angiotensin ii radioimmunoassay. Endocrinol Jpn 1985; 32: 803–809.
Sakata S, Tsuchihashi T, Oniki H, Tominaga M, Arakawa K, Sakaki M, Kitazono T . Relationship between salt intake as estimated by a brief self-administered diet-history questionnaire (bdhq) and 24- h urinary salt excretion in hypertensive patients. Hypertens Res 2015; 38: 560–563.
Acknowledgements
KFG is supported as a doctoral fellow CAPES foundation (process 2446/14-6); MP is supported by the Brazilian Council for the Scientific and Technological Development (CNPq, process 303566/2013-2); VAC is supported as a postdoctoral research fellow by research foundation Flanders (FWO) and by a grant of Krediet aan Navorsers (FWO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Supplementary information
Rights and permissions
About this article
Cite this article
Goessler, K., Polito, M. & Cornelissen, V. Effect of exercise training on the renin–angiotensin–aldosterone system in healthy individuals: a systematic review and meta-analysis. Hypertens Res 39, 119–126 (2016). https://doi.org/10.1038/hr.2015.100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.100
Keywords
This article is cited by
-
Effect of exercise training on the renin–angiotensin–aldosterone system: a meta–analysis
Journal of Human Hypertension (2023)
-
Lifestyle Medicine as a Treatment for Resistant Hypertension
Current Hypertension Reports (2023)
-
Interstitial-fluid shear stresses induced by vertically oscillating head motion lower blood pressure in hypertensive rats and humans
Nature Biomedical Engineering (2023)
-
Could Repeated Cardio-Renal Injury Trigger Late Cardiovascular Sequelae in Extreme Endurance Athletes?
Sports Medicine (2022)
-
Lifestyle interventions for the prevention and treatment of hypertension
Nature Reviews Cardiology (2021)