Abstract
Many ectothermic species are currently expanding their geographic range due to global warming. This can modify the population genetic diversity and structure of these species because of genetic drift during the colonization of new areas. Although the genetic signatures of historical range expansions have been investigated in an array of species, the genetic consequences of natural, contemporary range expansions have received little attention, with the only studies available focusing on range expansions along a narrow front. We investigate the genetic consequences of a natural range expansion in the Mediterranean damselfly Coenagrion scitulum, which is currently rapidly expanding along a broad front in different directions. We assessed genetic diversity and genetic structure using 12 microsatellite markers in five centrally located populations and five recently established populations at the edge of the geographic distribution. Our results suggest that, although a marginal significant decrease in the allelic richness was found in the edge populations, genetic diversity has been preserved during the range expansion of this species. Nevertheless, edge populations were genetically more differentiated compared with core populations, suggesting genetic drift during the range expansion. The smaller effective population sizes of the edge populations compared with central populations also suggest a contribution of genetic drift after colonization. We argue and document that range expansion along multiple axes of a broad expansion front generates little reduction in genetic diversity, yet stronger differentiation of the edge populations.
Similar content being viewed by others
Introduction
The causes and genetic consequences of range limits are key issues in ecology and evolution (Bridle and Vines, 2007; Gaston, 2009a) that directly relate to environmental challenges such as biological invasions, habitat loss, climate change and emerging diseases (Gaston, 2009b). The current climate change is causing dramatic changes in species distributions (David and Shaw, 2001) and has revived the interest in the evolutionary and ecological role of edge populations. Many ectothermic species are currently expanding their geographic range northwards (Hickling et al., 2005; Parmesan, 2006), which makes for exciting natural evolutionary experiments and offers unique opportunities to study evolutionary processes in edge populations. Although it is expected that global warming will continue to affect species’ ranges, few empirical studies have focused on the genetic consequences of contemporary, natural range expansion (Pauls et al., 2013).
The random genetic drift that accompanies the founding of new populations at the edge of the expansion front is known as the founder effect (Holgate, 1966; Hartl and Clark, 2007). Founder effects may leave a strong genetic signature in the newly established populations by reducing genetic diversity within populations while increasing genetic differentiation among populations (Nei et al., 1975; Excoffiier et al., 2009). Most of our insights on the genetic consequences of range expansions come either from historical (postglacial) range expansions (reviewed in Eckert et al., 2008) or from contemporary range expansions as a consequence of anthropogenic introductions (reviewed in Dlugosch and Parker, 2008), while natural range expansions may have a different genetic signature (McInerny et al., 2009; Hill et al., 2011). In contrast to what is typically assumed for historical range changes, potential distributions of species are currently changing at a very fast rate (Davis and Shaw, 2001; Malcolm et al., 2002; Chen et al., 2011), while contemporary natural range expansions differ from anthropogenic introductions as the latter are often accompanied with long distance dispersal events, anthropogenic introductions from different source populations (Dlugosh and Parker, 2008) and growing populations (McInerny et al., 2009). Understanding the genetic processes occurring during range expansion is important as these can drive widely different evolutionary consequences. For example, a decrease in genetic diversity in edge populations could impede adaptation to new environmental conditions (Bridle and Vines, 2007; Agashe et al., 2011), whereas enhanced genetic drift could accelerate or impede adaptation due to alleles surfing on the expansion front (Klopfstein et al., 2006; Burton and Travis, 2008).
Despite its relevance, only very recently the genetic consequences of natural, contemporary range expansions have started to receive attention (Watts et al., 2010; Banks et al., 2010; Garroway et al., 2011; Buckley et al., 2012). Results so far are equivocal. To our knowledge, only two studies reported the expected reduction in genetic diversity (Watts et al., 2010; Garroway et al., 2011), and only one the expected increase in genetic differentiation (Watts et al., 2010). These studies all documented range expansions occurring along a single axis of a relative narrow (<100 km) expansion front. However, in many systems there are several independent axes of expansion associated with independent expansion fronts (for example, Hughes et al., 2003) or associated with different directions along a broad expansion front (for example, Phillips and Shine, 2004). The latter types of range expansion may not only generate more independently replicated edge populations but likely also result in different genetic signatures along the expansion front. The establishment of edge populations at a broad expansion front with expansions in multiple directions likely results in (1) smaller reductions in genetic diversity as founders may derive from a broader set of core populations and (2) stronger genetic differentiation given the more independent expansion axes along the broad front, unless there is a high propagule pressure (Lockwood et al., 2005).
Here, we investigate the genetic signature of a natural, rapid range expansion in a damselfly species where the edge populations are situated at the western, northern and eastern parts of a broad expansion front. This allows investigating the genetic consequences using replicated edge populations along directions other than the North–South direction, thereby avoiding potential confounding effects of latitude (Johansson et al., 2006; Eckert et al., 2008) when comparing edge and core populations (Hassall and Thompson, 2008). Among a broad array of studied vertebrates and invertebrates, damselflies are showing some of the strongest range shifts under global warming (Hickling et al., 2005, 2006). Specifically, we here compare genetic diversity and genetic differentiation in edge populations and core populations of the damselfly C. scitulum (Rambur, 1842), a Mediterranean species expanding its range in North-western Europe (Wasscher and Goudsmits, 2010). In 10 years time, this species has established populations >100 km northeastward from its historical range (Wasscher and Goudsmits, 2010).
Materials and Methods
Study species and sample collection
The damselfly C. scitulum has a Mediterranean distribution (Dijkstra and Lewington, 2006). In the early nineties, the northern edge of its range was situated in Northern France (Dommanget et al., 1994). The species has recently expanded and founded populations in three directions: eastward (Switzerland: 2006 (Hoess, 2007)), northward (Belgium: 1998 (Wasscher and Goudsmits, 2010)), Germany: 2006 (Glitz, 2008), The Netherlands: 2007 (Geraeds et al., 2011), and westward (Jersey in 2009 (Perchard and Long, 2009) and mainland UK in 2010 (Parr, 2011)) (Figure 1). Although different factors can be causing this range expansion, it is known that many Southern Odonate species, besides C. scitulum, are currently expanding their range, suggesting a role for global warming (Hickling et al., 2005; Goffart, 2010; Parr, 2010; Termaat et al., 2010). The alternative explanation that C. scitulum is responding to improved habitat quality seems less likely as the species does not have a strong habitat preference (Wasscher and Goudsmits, 2010).
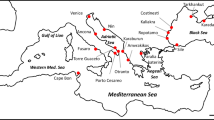
Map of the five core and five edge study populations of C. scitulum and the larger European distribution (inset). The year at which each edge population was first recorded is indicated. Population codes are explained in Table 1. The shaded area represents the historical range based on Dommanget et al., 1994; Dijkstra and Lewington, 2006 and Boudot, 2013 (unpublished data).
To capture the genetic signature of this range expansion we sampled various edge and core populations (Figure 1). In order to gauge independent populations, we sampled in the three directions of the broad front. Note that our sampling design was not chosen to compare the genetic signature among the different directions of the range front (which would need replicated edge populations in each direction, which is not possible given that there was at the time of sampling, for example, only one known population in the westward direction), but to capture the general genetic signature of the edge populations of the broad front using independent populations. Five edge and five core populations were sampled. Per population we collected between 30 and 32 individuals, except for Jersey (UK) where the only known population was small and where we only could obtain 13 animals (total number of animals=297). We defined core populations as those populations situated within the historical continuous distribution of the species, irrespective how close they are to the range margin. Edge populations were defined as those younger than 5 years and situated outside the continuous distribution of the species at the expansion front (Table 1; Figure 1). Note that stable edge populations are not considered as edge populations within our definition. The inclusion of core populations close to the historic range allows for a conservative approach in detecting the signature of recent range expansion. We only considered core populations from Western Europe (France and North Spain) and not from Central Europe (for example, Italy) because a broader geographic survey across Europe showed populations in Western Europe to be the most likely source of migrants for the studied edge populations (Swaegers et al., unpublished data).
DNA extraction and microsatellite genotyping
The DNA was isolated from legs using the nucleospin extraction kit (Machery Nagel, Düren, Germany). The DNA of the SLF population was extracted using a Chelex method (see Johansson et al., 2012). We genotyped all damselflies at 12 microsatellite markers (cosci_05, cosci_07, cosci_27, cosci_38, cosci_01, cosci_08, cosci_15, cosci_25, cosci_34, cosci_37, cosci_46, C2_1050) described in Johansson et al. (2012) and Swaegers et al. (2012). The genotyping protocols are outlined in Swaegers et al. (2012). An ABI 3130-Avant Genetic Analyzer was used to genotype all samples, together with a GENESCAN 500-LIZ internal size standard. The electropherograms were analyzed by the software program GENEMAPPER v 4.0 (Applied Biosystem, Foster City, CA, USA). To determine the genotyping error rate, we blindly repeated the genotyping of 20 samples (7% of total) starting from the DNA extract. The mean allelic error rate was found to be 0.001%.
Past and current genetic diversity
In a preliminary step, all populations were tested for Hardy–Weinberg equilibrium and linkage equilibrium using GENEPOP (Rousset, 2008), for null-alleles using MICRO-CHECKER (Van Oosterhout et al., 2004).
To test for the expected lower current genetic diversity in the edge populations compared with the core populations, we calculated four genetic diversity indices using the program GenAlEx 6.4 (Peakall and Smouse, 2006): expected and observed heterozygosity, number of alleles and the number of private alleles. As only 13 individuals of population UKJ (Jersey) could be collected compared with 30–32 individuals in the other populations (Table 1), we corrected for sample size when comparing the number of alleles by using rarefaction with the software ADZE (Szpiech et al., 2008). The size of the subsample was set to 22 haploid genomes. To test the prediction under the hypothesis of Nei et al. (1975) and Excoffier et al. (2009), namely that the values of the first three indices of genetic diversity are lower in edge populations than in core populations, we performed a one-sided permutation test (10 000 permutations) implemented in FSTAT for every index (Goudet, 1995).
Besides comparing current measures of genetic diversity, we also searched for genetic signatures of bottlenecks in the recent past. If founder effects have taken place during the range expansion we expect to detect signatures of bottlenecks in the edge populations. Their presence was evaluated using the software BOTTLENECK (Cornuet and Luikart, 1996). Given the recent nature of the current founder effects, if any, we only used the infinite allele model to detect this heterozygosity excess. Although typically explicit microsatellite mutation models are used for this test, mutations that have accumulated locally in the newly established populations will so far have had a negligible impact on the allelic richness and diversity.
Furthermore, we can expect that the number of alleles (k) is reduced faster than the overall range in allele size (r) in recently bottlenecked populations (Garza and Williamson, 2001). We, therefore, tested whether edge populations showed higher values of the so-called M-ratio (k/r, Garza and Williamson, 2001) compared with core populations. This was done by calculating the M-ratio for every population and testing for effects of population status (core vs edge) and locus using a general linear mixed model in SAS 9.3 where we included locus and population nested in population status as random factors.
To evaluate the amount of genetic drift in both edge and core populations, we estimated effective population sizes (Ne values) using Colony 2.0.1.8 by means of a sibship assignment method where the amount of relatedness was calculated assuming random mating and female and male polygamy (Wang, 2009). The estimated Ne values were compared between edge and core populations using a Mann–Whitney U-test.
Genetic structure and genetic differentiation
To infer the genetic structure of the samples and test for genetic differentiation between edge and core populations we used three approaches: (1) a Bayesian individual assignment method, (2) a factorial correspondence analysis (CA) and (3) regular and Bayesian measured summary indices at the population level. Firstly, to investigate whether edge populations indeed form a single evolutionary unit with core populations, the genetic structure in our data was analyzed based on Bayesian individual assignment using STRUCTURE 2.2 (Pritchard et al., 2000). This program traces the number of genetic clusters that minimizes Hardy–Weinberg and linkage disequilibrium using a Markov chain Monte-Carlo method. Both models with admixture and without admixture were used in the simulations. As dispersal can be expected to be high in damselfly species (Conrad et al., 1999; De Block et al., 2008; Shama et al., 2011), the former is considered more plausible. Correlated allele frequencies were assumed and the admixture model simulations were run with and without prior information on the population of the individuals. Runs were performed using a burn-in period of 20 000 replicates and a sampling period of 100 000 replicates. Simulations were run for a number of clusters (K) ranging from one to ten (the actual number of populations), and 20 iterations were performed for each K. The ln likelihoods from these iterations were averaged for each K and the most likely K was selected with the delta K method (Evanno et al., 2005).
Secondly, CA, a CA was performed on the individual multilocus genotypes to visualize the genetic structure between individuals of all populations using the software GENETIX (Belkhir et al., 2002). Thirdly, to explicitly test for stronger genetic differentiation in edge vs core populations, population-specific Fst values were estimated in a Bayesian framework using the program GESTE 2 (Foll and Gaggiotti, 2006). These population-specific Fst values indicate how genetically distinct the population is from the remainder of the metapopulation (Foll and Gaggiotti, 2006). The effect of population status on these Fst values was evaluated using generalized linear models in GESTE 2. Geographic effects were investigated by including latitude and longitude as explanatory variables in the models. The posterior probabilities of each possible model were compared to select the model with the highest probability given our data (Foll and Gaggiotti, 2006). To investigate pairwise genetic differentiation between populations, we calculated both Fst and D (Jost, 2008) averaged over all loci for every pair of populations using GENETIX (Belkhir et al., 2002) and DEMEtics (Gerlach et al., 2010), respectively, and tested for significance with 1000 permutations (in GENETIX) or bootstraps (in DEMEtics).
We tested for isolation by distance using Mantel tests implemented in the R package vegan 2.0-2 (Dixon, 2003). We tested this firstly using all populations and secondly using only the subset of core populations, as these populations can be expected to be nearer to migration-drift equilibrium due to their longer existence and more central location in the geographical range (Hutchison and Templeton, 1999). Isolation by distance was also tested using a distance-based redundancy analysis (Legendre and Fortin, 2010) in CANOCO for Windows 4.02 (Ter Braak, 1988) using the coordinates and orthogonal polynomials of the second grade (Stats v 2.15.1 R package, R Core Team) as explaining variables. Forward selection was performed using 9999 Monte-Carlo permutations. Subsequently, the effect of the three types of population status comparisons (edge–edge, edge–core or core–core) on their genetic differentiation (Fst or D) was tested using a general linear model after accounting for the geographic distance between population pairs. If genetic drift in edge populations is indeed important, comparisons involving edge populations should be stronger than those without them. As the population pairs are not independent, we used a resampling procedure (agricolae package in R 2.8.1, R Development Core Team 2009) to assess the significance of the effect (Vandewoestijne and Van Dyck, 2010).
Finally, we tested whether populations closer to the historic range margin displayed lower genetic diversity and higher genetic differentiation as has been proposed in the central-marginal hypothesis (Eckert et al., 2008). For every population, we measured the distance to the historic range margin and we tested for an association with the genetic indices using general linear models in R (for the analyses of genetic diversity) and GESTE 2 (for the Fst analysis). We did the analyses both with the distances of the edge populations considered as negative and as positive distances, yet this did not differ the qualitative outcome of the analyses (only results with negative distances reported).
Results
Current and past genetic diversity
Not all populations were found to be in Hardy–Weinberg equilibrium at each locus (after Bonferroni correction). Four populations showed significant homozygote excess at one locus (BPM: cosci_34, SC: cosci_08, SLF: cosci_15 and NV: cosci_34) and these deviations could be ascribed to the potential presence of null-alleles. As there was no association between these excesses and any population or locus, we retained all loci for further analysis. None of the locus pairs showed significant evidence of linkage disequilibrium after correction for multiple tests (Padjusted>0.05).
Expected heterozygosity (He) ranged from 0.62 in population PR to 0.67 in populations HWP and DNZ while observed heterozygosity (Ho) ranged from 0.55 in population SLF to 0.65 in population HWP (Table 1). The highest standardized number of alleles (after correcting for sample size, AR) was found in the SLF population (6.14) and the lowest mean number of alleles in population UKJ (5.23). The standardized number of private alleles in a population varied between 0.29 and 0.73 (Np, Table 1). No significant decrease in expected (P=0.248) and observed heterozygosity (P=0.412) was detected in the edge populations compared with the core populations. A marginally significant decrease in standardized number of alleles was found in the edge populations (P=0.047, Table 1).
A significant excess in expected heterozygosity was detected in the edge populations HWP (P=0.032), UKJ (P=0.017) and core population FRR (P=0.021). We found no evidence for a difference of the M-ratio statistic between core (mean±1 s.e.: 0.41±0.03) and edge populations (0.40±0.04) (F1, 8=0.2, P=0.664). The estimated Ne values of the edge populations (median=39, range=13) were significantly lower than those of the core populations (median=48, range=8) (Table 1, U=0.5, n=10, P=0.008).
Genetic structure and genetic differentiation
The analysis using STRUCTURE could not detect any clustering in the sampled populations of C. scitulum. Both under the assumption of admixture with or without prior information as under the assumption of no admixture, the highest likelihood of the data was found in the model with K=1 (suggesting a common ancestry of all populations). However, genetic structure was apparent from the CA (Figure 2). The first axis explained 18.8% of the variance among populations, the second axis explained 14.6% and the third axis explained 13.2%. As axis 2 mostly explained variation between the Jersey (UKJ) population and the other populations, this axis was, therefore, not as informative for the general pattern between edge and core populations. A plot of axes 1 and 3 showed that most of the core populations are aggregated in the center of the plot, whereas most edge populations are spread around this cluster (Figure 2). Exceptions are the Swiss SC edge population, which clusters with the core populations and the Spanish SLF core population, which distinguishes itself from the other four core populations.
2D plot of axes 1 and 3 of the factorial CA showing genetic structure between the five core and five edge study populations of C. scitulum. Population codes are explained in Table 1. Axis 2 is not shown, as it mostly explains the separation of Jersey from all other populations.
The Bayesian analysis in GESTE 2 also indicated the structuring role of population status. Strongest support was found for the model containing only the factor population status (Table 2). Population-specific Fst values were greater in edge populations than in core populations (Table 1, population status coefficient: 0.551; 95% highest probability density interval: (0.0948, 1.01)). The analysis with GESTE 2 did not detect any influence of longitude or latitude on the population-specific Fst values. Looking at pairwise genetic distances between populations, also the Mantel tests did not reveal any association of genetic distances with geography: low, non-significant positive correlations between geographic distance and Fst (Mantel r=0.167, P=0.139) or D (Mantel r=0.095, P=0.255) were inferred, indicating the absence of (strong) isolation by distance. The analysis using distance-based redundancy analysis generated similar results (P>0.508). When only analyzing the subset of core populations, no signal of isolation by distance could be found either (Fst: Mantel r=0.153, P=0.436; D: Mantel r=−0.110, P=0.647).
The pairwise Fst values ranged from 0.001 to 0.055 (Table 3). Overall, D values were higher with a range from 0.004 to 0.164, as expected with highly variable markers (Table 3). For both Fst and D, 28 out of 45 comparisons were statistically significant after a Bonferroni correction for multiple comparisons (Padjusted<0.05). For every edge population, significant pairwise differentiations were found in both edge–edge as edge–core comparisons, except for the Swiss edge population SC, where the comparisons with the core populations were not significant. In contrast, none of the core–core (CC) comparisons showed significant differentiation when expressed as D. The same trend was observed for Fst except for the Spanish core population SLF, where a significant differentiation was found with the French core populations BPM and PR. These results were confirmed in an overall general linear model that corrected for geographic distances: the type of population status comparison had a significant effect on the degree of genetic differentiation both expressed as Fst (F1,43=14.46, Presampling=0.002) and as D (F1,43=24.19, Presampling<0.001) (Figure 3). Tukey posthoc tests revealed that the genetic differentiation expressed as Fst was significantly higher between two edge populations than between two core populations (edge–edge vs CC: Padjusted=0.001), while the genetic differentiation between a core and an edge population was intermediate and not different from the other two types (both P>0.05). The pattern was very similar when based on D values, with the difference that the intermediate genetic differentiation between edge and core populations did significantly differ from the other two groups (edge–edge vs edge–core: Padjusted =0.030; edge–core vs CC: Padjusted=0.008).
No significant effects of the distance to the historic range on allelic richness (t8=1.59, P=0.151), expected heterozygosity (t8=−0.43, P=0.677) and the population-specific Fst values (model with the highest likelihood contained only a constant) were found (Figure 4).
Discussion
Our key findings were in line with our expectations under a scenario where a rapid, natural range expansion occurs along a broad expansion front involving many dispersers. We could detect a significant difference in the degree of genetic differentiation between the core and edge populations and a marginal significant difference in allelic richness, indicating that the number of sampled populations, individuals and loci were sufficient to detect genetic signatures of recent range expansion.
The slight decrease in allelic richness in the newly founded edge populations, yet not in heterozygosity is expected as allelic richness is more sensitive to founder effects than heterozygosity (Nei et al., 1975). This pattern is also commonly found in invasive species (Dlugosh and Parker, 2008). The finding that the decrease in genetic diversity is rather subtle is indicative of a range expansion where edge populations are established from a high number of individuals and possibly multiple colonization waves. This is a realistic scenario in our case as the possible migration routes from different core regions and the high dispersal capability commonly observed in damselflies (Conrad et al., 1999; De Block et al., 2008; Shama et al., 2011) could allow for a high migration rate. Unfortunately, the general low genetic differentiation between our populations did not allow estimating migration rates between populations. Simulation models have shown that when new populations are founded by a high number of migrants and the subsequent migration rate between core and edge population is high, the founder effect is negligible (Nei et al., 1975; Le Corre and Kremer, 1998). This way, the effects of climate-induced range expansion on the genetic diversity of the edge populations can be minor. Although a decrease in allelic richness has been detected in edge populations in two studies (Watts et al., 2010; Garroway et al., 2011), all previous studies that have investigated genetic patterns of recent climate-induced range expansion also conclude that expansion occurred in a continuous wave of expansion with extensive gene flow (Watts et al., 2010; Dawson et al., 2010; Banks et al., 2010; Garroway et al., 2011; Buckley et al., 2012). In addition, the bottleneck analysis did not reveal a clear pattern of edge populations undergoing bottlenecks; instead we found evidence for a bottleneck in the recent past in two edge populations and one core population. For edge populations HWP (the Netherlands) and UKJ (Jersey), this might mean that fewer colonizers have established these populations. The bottleneck in the core population FRR (France) could be the consequence of local drift processes that have acted in the recent past.
Our data indicate an increase in genetic differentiation during range expansion, with edge populations being more differentiated relative to those in the core range. Although we did not find a decrease in heterozygosity in the edge populations, allele frequencies significantly differed more in edge populations. Moreover, the pairwise Fst values indicated more genetic differentiation among the edge populations. Importantly, this pattern remained when correcting for the geographic distances between the populations. In comparison with the classical pairwise Fst measures, the pattern was more apparent with the estimated pairwise D values. This was expected as D has a greater capacity to detect genetic differentiation when using highly variable genetic markers such as microsatellite markers (Jost, 2008; Leng and Zhang, 2011; Raeymaekers et al., 2012).
An increase in genetic differentiation during range expansion is consistent with four evolutionary scenarios (Beaumont and Nichols, 1996; Eckert et al., 2008; Excoffier et al., 2009; Watts et al., 2010): (1) genetic drift during range expansion, that is, the founder effect, (2) genetic drift after the establishment of the edge populations (that is, low Ne), (3) selection acting on the genetic markers and (4) the foundation of edge populations from different genetically structured source populations. The latter scenario seems unlikely, as core populations could not be distinguished from each other neither by the STRUCTURE analysis nor visually in the CA plot (except for the Spanish SLF population). Also, selection on the genetic markers or linked loci (scenario 3) is no plausible explanation for the elevated genetic differentiation at the edge, as none of the genetic markers displayed an extreme Fst value between edge and core populations compared with the other markers (data not shown). Our results, on the contrary, suggest a role for genetic drift in explaining the pattern: individuals from all edge populations in the CA plot (except for the Swiss SC population) are situated in different directions away from the center where the core populations are situated indicating random allele frequency changes in the microsatellite markers and hence a founder effect (scenario 1). The finding that effective population sizes are significantly smaller in edge populations compared with core populations also suggests that the presence of genetic drift after colonization (scenario 2) may contribute to the observed patterns. The higher spread of individuals within edge populations compared with the individuals within central populations in the CA plot also suggests the presence of genetic drift after colonization. When a population goes through a bottleneck (a reduction in Ne compared with its original state), allelic richness decreases more rapidly than the expected heterozygosity (Luikart and Cornuet, 1998). Such a scenario is also supported by the slight but significant reduction in standardized number of alleles, but not in expected heterozygosity, in edge populations. Hence, it is likely that both founder effects and subsequent genetic drift affected population genetic structure. Alternatively, smaller Ne could be due to lower quality of the habitat at the edge of the distribution (Eckert et al., 2008). It might be that the Swiss SC edge population has experienced less genetic drift compared with the other edge populations due to its relatively older age and vicinity to the historical range, resulting in a similar genetic composition as the core populations. The Spanish SLF core population, on the other hand, might be isolated due to the Pyrenean mountains forming a barrier for gene flow, hence resulting in a deviant genetic composition than the other core populations, as recently shown for another Coenagrion damselfly species (Johansson et al., 2013).
From the five other studies investigating the genetic consequences of recent, natural range expansions (Table 4), an increase in genetic differentiation in the edge populations has only been detected in the study by Watts et al., 2010. In their study, it is mentioned that the contributions of genetic drift during range expansion (scenario 1) and source genomic structure (scenario 4) were confounded as not enough source populations were sampled. The strong indications of genetic differentiation during range expansion in our study is in line with our prediction that such genetic signature is likely to be stronger in expansions along multiple axes at a broad front, where gene flow possibilities between edge populations are more limited.
Despite the large number of species showing range expansions in response to global warming (Hickling et al., 2005, 2006; Parmesan, 2006), and the enormous impact this may have on biodiversity patterns (Bellard et al., 2012); surprisingly few studies investigated the genetic consequences of such range expansions (Table 4). Yet, this is highly relevant as it will contribute to our understanding of the ability of newly founded populations to locally adapt, hence persist, and even evolve higher dispersal abilities which on its turn will determine the speed of further range expansion (Hughes et al., 2003; Bridle and Vines, 2007; Hill et al., 2011). We here proposed and documented that a range expansion along multiple axes along a broad expansion front is associated with little reduction in genetic diversity, yet stronger differentiation in the edge vs the core populations. More studies on the genetic effects of range expansion are needed to help decide, which characteristics (such as the width of the expansion front) are essential to generate a predictive framework for the genetic consequences of range expansions.
Data Archiving
Data deposited in the Dryad repository: doi:10.5061/dryad.d21t0.
References
Agashe D, Falk JJ, Bolnick DI . (2011). Effects of founding genetic variation on adaptation to a novel resource. Evolution 65: 2481–2491.
Banks SAMC, Ling SD, Johnson CR, Piggott MP, Jane E . (2010). Genetic structure of a recent climate change-driven range extension. Mol Ecol 19: 2011–2024.
Beaumont MA, Nichols RA . (1996). Evaluating loci for use in the genetic analysis of population structure. Proc Royal Society B 263: 1619–1626.
Belkhir K, Borsa P, Chikhi L, Raufaste N, Bonhomme F . (2002). GENETIX 4.04, logiciel sous Windows TM pour la génétique des populations. Laboratoire Génome, Populations, Interactions, CNRS UMR 5000,Université de Montpellier II,Montpellier.
Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F . (2012). Impacts of climate change on the future of biodiversity. Ecol Lett 15: 365–377.
Bridle JR, Vines TH . (2007). Limits to evolution at range margins: when and why does adaptation fail? Trend Ecol Evol 22: 140–147.
Buckley J, Butlin RK, Bridle JR . (2012). Evidence for evolutionary change associated with the recent range expansion of the British butterfly, Aricia agestis, in response to climate change. Mol Ecol 21: 267–280.
Burton OJ, Travis JMJ . (2008). Landscape structure and boundary effects determine the fate of mutations occurring during range expansions. Heredity 101: 329–340.
Conrad KF, Willson KH, Harvey IF, Thomas CJ, Sherratt TN . (1999). Dispersal characteristics of seven odonate species in an agricultural landscape. Ecography 22: 524–531.
Cornuet JM, Luikart G . (1996). Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144: 2001–2014.
Davis MB, Shaw RG . (2001). Range shifts and adaptive responses to Quaternary climate change. Science 292: 673–679.
Dawson MN, Grosberg RK, Stuart YE, Sanford E . (2010). Population genetic analysis of a recent range expansion: mechanisms regulating the poleward range limit in the volcano barnacle Tetraclita rubescens. Mol Ecol 19: 1585–1605.
De Block M, Geenen S, Jordaens K, Backeljau T, Stoks R . (2008). Spatiotemporal allozyme variation in the damselfly, Lestes viridis (Odonata: Zygoptera): gene flow among permanent and temporary ponds. Genetica 124: 137–144.
Dijkstra KDB, Lewington R . (2006) Field Guide to the Dragonflies of Britain and Europe. British Wildlife Publishing.
Dixon P . (2003). VEGAN, a package of R functions for community ecology. J Vegetation Sci 14: 927–930.
Dlugosch KM, Parker IM . (2008). Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17: 431–449.
Dommanget JL, Procida G, Brulard JF, Da Costa H, Haffner P . (1994). Atlas préliminaire des Odonates de Frances. Secretariat de la Faune et de la Flore (Hrsg.): Collection Patrimoines naturels (Paris) 16: 1–92.
Eckert CG, Samis KE, Lougheed SC . (2008). Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Mol Ecol 17: 1170–1188.
Evanno G, Regnaut S, Goudet J . (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14: 2611–2620.
Excoffier L, Foll M, Petit RJ . (2009). Genetic Consequences of Range Expansions. Annual Review of Ecology, Evolution, and Systematics 40: 481–501.
Foll M, Gaggiotti O . (2006). Identifying the environmental factors that determine the genetic structure of populations. Genetics 174: 875–891.
Goffart P . (2010). Southern dragonflies expanding in Wallonia (south Belgium): a consequence of global warming? BIORISK—Biodiversity and Ecosystem Risk Assessment 5: 109–126.
Garroway CJ, Bowman J, Holloway GL, Malcolm JR, Wilson PJ . (2011). The genetic signature of rapid range expansion by flying squirrels in response to contemporary climate warming. Global Change Biol 17: 1760–1769.
Garza JC, Williamson EG . (2001). Detection of reduction in population size using data from microsatellite loci. Mol Ecol 10: 305–318.
Gaston KJ . (2009a). Geographic range limits: achieving synthesis. Proc Royal Society B 276: 1395–1406.
Gaston KJ . (2009b). Geographic range limits of species. Proc Royal Society B 276: 1391–1393.
Geraeds R, Hermans J, Ramaker A . (2011). [De Gaffelwaterjuffer opnieuw in Limburg gevonden]. Natuurhistorisch Maandblad 100: 41–45.
Gerlach G, Jueterbock A, Kraemer P, Deppermann J, Harmand P . (2010). Calculations of population differentiation based on Gst and D: forget Gst but not all of statistics!. Mol Ecol 19: 3845–3852.
Glitz D . (2008). Erstnachweis von Coenagrion scitulum in Rheinland-Pfalz (Odonata: Coenagrionidae). Libellula 27: 33–37.
Goudet J . (1995). FSTAT (Version 1.2): A computer program to calculate F-statistics. J Hered 86: 485–486.
Hartl DL, Clark AG . (2007) Principles of Population Genetics. Sinauer Associates Inc. Publishers, USA.
Hassall C, Thompson DJ . (2008). The effects of environmental warming on Odonata: a review. Int J Odonatol 11: 131–154.
Hickling R, Roy DB, Hill JK, Thomas CD . (2005). A northward shift of range margins in British Odonata. Global Change Biol 11: 502–506.
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD . (2006). The distributions of a wide range of taxonomic groups are expanding polewards. Global Change Biol 12: 450–455.
Hill JK, Griffiths HM, Thomas CD . (2011). Climate change and evolutionary adaptations at species’ range margins. Ann Rev Entomol 56: 143–159.
Hoess R . (2007). [War Coenagrion scitulum (Rambur, 1842) (Odonata: Coenagrionidae) Einst in der Schweiz heimisch?]. Mitteilungen der Entomologischen Gesellschaft Basel 57: 2–9.
Holgate P . (1966). A mathematical study of the founder principle of evolutionary genetics. J Appl Prob 3: 115–128.
Hughes CL, Hill JK, Dytham C . (2003). Evolutionary trade-offs between reproduction and dispersal in populations at expanding range boundaries. Proc Royal Society B 270: S147–S150.
Hutchison D, Templeton A . (1999). Correlation of pairwise genetic and geographic distance measure: inferring the relative influences of gene flow and drift on distribution of genetic variability. Evolution 53: 1898–1914.
Johansson H, Ingvarsson PK, Johansson F . (2012). Cross-species amplification and development of microsatellites for six species of European Coenagrionid damselflies. Conservation Genetics Resources 4: 191–196.
Johansson H, Stoks R, Nilsson-Örtman V, Ingvarsson P, Johansson F . (2013). Large-scale patterns in genetic variation, gene flow and differentiation in five species of European Coenagrionid damselfly provide mixed support for the central-marginal hypothesis. Ecography 36: 744–755.
Johansson M, Primmer CR, Merilä J . (2006). History vs. current demography: explaining the genetic population structure of the common frog (Rana temporaria). Mol Ecol 15: 975–983.
Jost L . (2008). GST and its relatives do not measure differentiation. Mol Ecol 17: 4015–4026.
Klopfstein S, Currat M, Excoffier L . (2006). The fate of mutations surfing on the wave of a range expansion. Mol Biol Evol 23: 482–490.
Le Corre V, Kremer A . (1998). Cumulative effects of founding events during colonisation on genetic diversity and differentiation in an island and stepping-stone model. J Evol Biol 11: 495–512.
Legendre P, Fortin M-J . (2010). Comparison of the Mantel test and alternative approaches for detecting complex multivariate relationships in the spatial analysis of genetic data. Mol Ecol Resources 10: 831–844.
Leng L, Zhang DE-X . (2011). Measuring population differentiation using Gst or D? A simulation study with microsatellite DNA markers under a finite island model and nonequilibrium conditions. Mol Ecol 20: 2494–2509.
Lockwood JL, Cassey P, Blackburn T . (2005). The role of propagule pressure in explaining species invasions. Trend Ecol Evol 20: 223–228.
Luikart G, Cornuet JM . (1998). Empirical evaluation of a test for identifying recently bottlenecked populations from allele frequency data. Conservation Biol 12: 228–237.
Malcolm JR, Markham A, Neilson RP, Garaci M . (2002). Estimated migration rates under scenarios of global climate change. J Biogeography 29: 835–849.
McInerny GJ, Turner JRG, Wong HY, Travis JMJ, Benton TG . (2009). How range shifts induced by climate change affect neutral evolution. Proc Royal Society B 276: 1527–1534.
Nei M, Maruyama T, Chakraborty R . (1975). The bottleneck effect and genetic variability in populations. Evolution 29: 1–10.
Parr A . (2010). Monitoring of odonata in Britain and possible insights into climate change. BIORISK—Biodiversity and Ecosystem Risk Assessment 5: 127–139.
Parr A . (2011). Migrant and dispersive dragonflies in Britain during 2010. J British Dragonfly Soc 27: 69–79.
Parmesan C . (2006). Ecological and evolutionary responses to recent climate change. Ann Rev Ecol, Evol System 37: 637–669.
Pauls SU, Nowak C, Bálint M, Pfenninger M . (2013). The impact of global climate change on genetic diversity within populations and species. Mol Ecol 22: 925–946.
Peakall R, Smouse PE . (2006). Genalex 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6: 288–295.
Perchard R, Long R . (2009). The rediscovery of Dainty Damselfly Coenagrion scitulum (Ramb.) in Jersey. Atropos 38: 3–5.
Phillips BL, Shine R . (2004). Adapting to an invasive species: toxic cane toads induce morphological change in Australian snakes. Proceedings of the National Academy of Sciences of the United States of America 101: 17150–17155.
Pritchard JK, Stephens M, Donnelly P . (2000). Inference of population structure using multilocus genotype data. Genetics 155: 945–959.
Raeymaekers JAM, Lens L, Van Den Broeck F, Van Dongen S, Volckaert FAM . (2012). Quantifying population structure on short timescales. Mol Ecol 21: 3458–3473.
Rousset F . (2008). GENEPOP ’ 007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol 8: 103–106.
Shama LNS, Campero-Paz M, Wegner KM, DE Block M, Stoks R . (2011). Latitudinal and voltinism compensation shape thermal reaction norms for growth rate. Mol Ecol 20: 2929–2941.
Shine R, Brown GP, Phillips BL . (2011). An evolutionary process that assembles phenotypes through space rather than through time. Proc Natl Acad Sci USA 108: 5708–5711.
Swaegers J, Mergeay J, Maes GE, Van Houdt JKJ, Larmuseau MHD, Stoks R . (2012). Microsatellite marker development and putative SNP detection for a northward expanding damselfly species using next generation sequencing. Conservation Genetics Resources 4: 1079–1084.
Szpiech ZA, Jakobsson M, Rosenberg NA . (2008). ADZE: a rarefaction approach for counting alleles private to combinations of populations. Bioinformatics 24: 2498–2504.
ter Braak CJF . (1988). CANOCOA:A FORTRAN Program for Canonical Community Ordination by [partial] [detrended] [canonical] Correspondence Analysis, Principal Component Analysis and Redundancy Analysis, Ver 2.1. LWA-88-02. Technical Report. DLO-Agricultural Mathematics Group, Wageningen, The Netherlands.
Termaat T, Kalkman V, Bouwman J . (2010). Changes in the range of dragonflies in the Netherlands and the possible role of temperature change. BIORISK—Biodiversity and Ecosystem Risk Assessment 5: 155–173.
Vandewoestijne S, Van Dyck H . (2010). Population genetic differences along a latitudinal cline between original and recently colonized habitat in a butterfly. PloS ONE 5: e13810.
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P . (2004). Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4: 535–538.
Wang J . (2009). A new method for estimating effective population sizes from a single sample of multilocus genotypes. Mol Ecol 18: 2148–2164.
Wasscher M, Goudsmits K . (2010). De gaffelwaterjuffer (Coenagrion scitulum), terug van weggeweest in Noordwest-Europa. Brachytron 13: 19–25.
Watts PC, Keat S, Thompson DJ . (2010). Patterns of spatial genetic structure and diversity at the onset of a rapid range expansion: colonisation of the UK by the small red-eyed damselfly Erythromma viridulum. Biol Invasions 12: 3887–3903.
Acknowledgements
We thank Bart Hellemans for technical assistance, Joost Raeymaekers for valuable discussions and Klaus-Jürgen Conze, Frank Johansson, Christian Monnerat, Viktor Nilsson-Örtman, Richard Perchard, Jochen Rodenkirchen, Sharon Schillewaert and Cédric Vanappelghem for their help with the collection of the samples. Jean-Pierre Boudot kindly provided recent European distribution data of the species. Two anonymous reviewers provided thoughtful comments that improved the presentation of the data. MHD Larmuseau received a postdoctoral fellowship of FWO-Vlaanderen. This study was funded by the KU Leuven Centre of Excellence Financing on ‘Eco- and socio-evolutionary dynamics’ (Project number PF/2010/07) and a research grant from the Fund for Scientific Research (FWO) Flanders.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Swaegers, J., Mergeay, J., Therry, L. et al. Rapid range expansion increases genetic differentiation while causing limited reduction in genetic diversity in a damselfly. Heredity 111, 422–429 (2013). https://doi.org/10.1038/hdy.2013.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2013.64
Keywords
This article is cited by
-
Genetic footprints of a rapid and large-scale range expansion: the case of cyclic common vole in Spain
Heredity (2023)
-
Invasion genetics of the Asian hornet Vespa velutina nigrithorax in Southern Europe
Biological Invasions (2022)
-
Geography alone cannot explain Tetranychus truncatus (Acari: Tetranychidae) population abundance and genetic diversity in the context of the center–periphery hypothesis
Heredity (2020)
-
Genetic structure of dioecious and trioecious Salix myrsinifolia populations at the border of geographic range
Tree Genetics & Genomes (2017)
-
Odonata (dragonflies and damselflies) as a bridge between ecology and evolutionary genomics
Frontiers in Zoology (2016)