Abstract
Populations of predominantly selfing plant species often show spatial genetic structure but little is known whether epistatic gene interactions are spatially structured. To detect a possible epistatic effect and a spatial scale at which it operates, we created artificial crosses between plants spanning a range of fixed distances from 1 to 400 m in three populations of wild barley. The self-pollinated and crossed progeny (F1) and two generations of segregated progeny (F2 and F3) were tested in experimentally simulated population environments for relative performance (RP). The measured fitness traits included number of seeds, total seed weight and seed germination. For any of these traits, there was no association between RP of F1, F2 and F3 plants and either pairwise kinship coefficients or crossing distance. In contrast, in all three populations, we found lower seed viability of outcrossed as compared with self-pollinated genotypes in the first generation of segregation. However, in the F3 generation this outbreeding effect disappeared in the two populations and greatly decreased in the third population. For seed production, heterosis in F1 and outbreeding depression in F2 were observed only in the population with unusually high number of heterozygotes. Our findings support the view that in selfing species a spatial mosaic of various locally abundant genotypes represents not randomly fixed combinations of alleles but the co-adapted gene complexes that were sieved by selection, while heterozygotes are characteristic for the transient phase of this process, when segregation and purging of maladaptive genotypes have not yet occurred.
Similar content being viewed by others
Introduction
Epistasis, defined as any form of gene interaction (Fisher, 1918) has been recognized for its importance in the evolution of natural populations (Whitlock et al., 1995; Wolf et al., 2000). Epistasis can be positive or negative depending on a relative fitness of the extreme as compared with the intermediate genotypes. The most powerful method for detecting epistasis is experimental hybridization of individuals from differentiated gene pools and analysis of the progeny (Fenster et al., 1997). Reduced fitness of progeny as compared with their parents, known as hybrid breakdown or outbreeding depression, evidences the positive epistatic effect. Alternatively, hybrid vigor (heterosis) can be detected in F1 hybrids as a result of overdominance or fixation of maladaptive genotypes under no gene flow.
Factors that limit or prevent recombination are expected to facilitate development of inter-locus interactions and creation of coadapted gene combinations. Selfing is known to reduce efficient recombination throughout the genome and to increase isolation between individuals and populations (Nordborg, 2000; Charlesworth, 2003). Although mating system is not a simple predictor of levels of linkage disequilibrium (Morrell et al., 2003, 2005), high linkage disequilibrium (LD) is an important feature of inbreeding species (for example, Nordborg et al., 2002; Garris et al., 2003; Zhu et al., 2003; Hamblin et al., 2004) that can lead to small effective sizes of local demes and spatial structure even within populations (Charlesworth, 2003; Siol et al., 2007). Therefore, selfing is one of the evolutionary avenues to development and maintenance of coadapted gene complexes through creation of epistatic combinations of alleles with improved fitness (Fenster et al., 1997). In contrast, the negative epistatic effect of creation of maladaptive genotypes through reduced gene flow and increased genetic drift, under selfing will also be reinforced (Kondrashov, 1988; Charlesworth, 1990). Spatial genetic structure is a common phenomenon in predominantly selfing plant species (Allard et al., 1972; Brown and Kodric-Brown, 1977; Golenberg, 1987; Bonnin et al., 2001; Siol et al., 2007, 2008), but little is known regarding the contribution of either negative or positive epistasis to this structuring.
In spite of a great increase in interest in understanding effects of crossing between genetically divergent lineages in the last decade (Hufford and Mazer, 2003; Tallmon et al., 2004; Edmands, 2007) very few studies of intraspecific hybridization in plants were conducted on selfing species, and usually at a spatial scale ranging from hundreds of meters to hundreds of kilometers (reviewed in Campbell and Waser, 2001; Edmands, 2007). However, spatial genetic structure was observed in autogamous or partially selfing plants at a much lower scale (Hamrick and Holden, 1979; Lonn, 1993; Williams, 1994; Volis et al., 2004a). There are three possible routes for divergent epistasis to evolve. The structure of genetically divergent neighborhoods can be a result of divergent selection resulting from spatial heterogeneity, for example, soil or microclimatic differences (Allard et al., 1972; Latta et al., 2004; Johansen-Morris and Latta, 2006; Ramakrishnan et al., 2006). Such structure can be also caused by genetic drift under limited seed and pollen dispersal (Williams, 1994; Siol et al., 2008), and strongly resemble the micro-environmental selection effect when seed dispersal and environmental heterogeneity occur at similar scale (Volis et al., 2004a). Finally, a mosaic of different genetic neighborhoods can result from a combination of uniform selection and drift. In the latter case, homogeneous environment selects for multiple solutions (‘adaptive peaks’) through creation of different coadapted gene combinations (Wright, 1931).
The vast majority of intraspecific hybridization studies crossed divergent ecotypes, or used geographic distance as a proxy to environmental differences between locations. In contrast, studies of uniform selection effects are very rare (Parker, 1992; Bieri and Kawecki, 2003). In this study, we were interested in distinguishing effects of genetic drift and uniform selection in creation of the spatial genetic structure under predominant self-pollination. We put forth the following alternative hypotheses: (i) in the case that spatial structure in a predominantly selfing plant species is a result of genetic drift under limited gene flow with fixation of random and often maladaptive gene combinations, then within-population hybridization events will lead to heterosis with no outbreeding depression in the following generations of selfing; (ii) if both genetic drift and natural selection operate, only the best fit gene combinations will become fixed in sub-populations, and hybridization will lead to outbreeding depression either in the F1 (no heterosis) or from the F2 generation onward (heterosis is present). A scale at which these processes are detectable is related to the distance of effective seed and pollen dispersal, and can be evident in a relationship of genetic relatedness with spatial distance. Outbreeding depression and heterosis are expected to occur in progeny of parents separated by the distance approximately equal or acceding effective genetic neighborhood, that is, the distance of limited seed and pollen flow. Under two scenarios of drift alone and drift with uniform selection both genetic relatedness and hybridization effects will correspond to the scale of limited gene flow, while under diversifying selection in a spatially heterogeneous environment they will be related to the scale of environmental heterogeneity. The latter scale must exceed the former one because otherwise homogenizing effect of gene flow will prevent local adaptation. Therefore, we designed a study to investigate the within-population hybridization (=outbreeding) effect and its relationship with genetic relatedness of the crossed parents in a predominant selfer at a small spatial scale ranging 1–400 m. This range of distances was chosen to embrace the possible scale of microhabitat differences in soil, topography or biotic interactions (Nevo et al., 1986, 2005; Huang et al., 2002) and range of gene flow (either through seed or pollen) (Wagner and Allard, 1991; Volis et al., 2010) in wild barley. Spatial environmental heterogeneity was not observed in the studied populations but could not be excluded as a possibility (for example, microclimate or spatially structured plant–pathogen interactions). We addressed the following questions: (1) does performance of crosses and progeny depend on genetic relatedness of the parents? (2) does performance of progeny change from F1 to F3 and what is the role of epistasis and heterosis in this process? and (3) is there any evidence for distance-dependent outbreeding depression?
Materials and methods
Study species and sampling
Hordeum spontaneum Koch is a predominantly selfing (>98%, Brown et al., 1978) winter annual grass widely distributed in Israel across different environments (Harlan and Zohary, 1966).
As a result of outcrossing effects, such as heterosis and outbreeding depression, may depend on the particular population history (that is, population size and its temporal fluctuations, plant density, patchiness of environment), and the observed heterozygosity, the plant material was collected at three locations in Israel separated by >100 km. These locations represent three distinct environments and vegetation communities: Mediterranean grassland (AM); batha (BG); and desert (SB) (for details, see Volis et al., 2002). At each location sampling was carried out during March–April 2001 in linear transects at fixed distances (0, 1, 2, 5, 10, 20, 50, 100 and 400 m, except for the BG site wherein the 400 m distance class was not collected). The nine transects within each location were separated by at least 100 m.
A single spikelet (=seed) was taken at each sample site. These spikelets were germinated and plants grown in a greenhouse in the following season. For genetic analysis, the leaves were collected at the two-leaf stage and stored under −20 °C. Plants grown from seeds collected from each plant were used in the crossing experiment.
Although no visible microhabitats were detected in any population environment and the populations were chosen paying special attention to homogeneity of their environments, presence of spatial heterogeneity that is not visible, and scale of this heterogeneity, can be revealed by spatial autocorrelation analysis that uses some measure of microsite quality. Soil nitrogen was used as such a proxi to soil spatial heterogeneity in numerous studies (for example, Lechowicz and Bell, 1991; Miller et al., 1995; Schlesinger et al., 1996). Specifically in wild barley, very large differences in soil nutrient status between sites of locally adapted populations were detected (Verhoeven et al., 2004) and selective role of nutrient status in population divergence was shown experimentally (Verhoeven et al., 2004; Volis et al., 2004b).
Therefore, to ensure that the populations occupy spatially homogeneous (that is, with no detectable microhabitats) environment, at each sampling point a soil sample was taken together with a spikelet. Spatial heterogeneity of three population environments was assessed by measuring nutrient availability in the soil. Available soil nitrogen (NO3−) was measured in the soil cores. The soil was sieved and 10 g (fresh weight) were processed according to standard protocols (Mulvaney, 1996). The three population locations had different soil type (loess, rendzina and Terra Rossa) and therefore differed in available nitrogen (18.7±2.9, 15.6±1.1 and 13.7±1.1 p.p.m., SB, BG and AM, respectively). However, no significant spatial autocorrelation of nitrogen availability was detected in any of the three populations. Although the autocorrelation coefficient for the first distance class of 1 m was positive, it was not statistically significant (r=0.088, 0.077, 0.194, P>0.05) for populations SB, BG and AM, respectively.
Crossing design
Within-transect crosses were performed in 2005 by artificial pollination of a ‘zero-distance’ mother plant with pollen of plants sampled at fixed distances from it (1, 2, 5, 10, 20, 50, 100 and 400 m) (P plants) (Figure 1). Crossing was carried out using a protocol developed for Hordeum vulgare. The procedure started with ear emasculation (that is, removal of immature anthers) at the bolting stage, when the awn emerged 3–5 cm from the leaf sheath but the inflorescences were still enclosed within the leaf sheath. Then the leaf sheath was folded back, enclosed in a paper bag, which was closed at base with a paperclip to prevent dehydration. Pollination was performed 3 days after emasculation when the flowers on the mother plant were open and the style at the base of the flower was clearly exposed. Mature anthers with pollen (which were yellow in contrast to the white anthers that had already released pollen) collected from the pollen donor, were deposited in each recipient flower. Then the pollinated ear was enclosed again within the paper bag, and marked with the date of pollination and the pollen donor plant.
In total, 198 crosses (81% of performed cross-pollinations) were successful. The produced hybrids (F1) and two generations of their self-pollinated offspring (F2 and F3) were analyzed for their relative performance (RP) as compared with the self-pollinated ″zero-plants″ in subsequent experiments (Figure 1).
Common garden experiments
The hybrids (F1), regarded as families of full-sibs nested within half-sibs families, and their self-pollinated offspring (F2) were grown together with plants derived from self-pollinated ‘zero-plant’ parent in two consecutive seasons, 2005–2006 and 2006–2007. The experiments had identical experimental design and differed only in number of plants used and amount of water supplied. Owing to the limited number of F1 seeds because of relatively low success of artificial pollination, it was not possible to plant simultaneously F1 and F2 generations. And because of the large number of families it was not possible to plant both mother and father plants. Therefore, our experimental design corresponded to a one-directional gene flow by pollen. Seeds were simultaneously germinated in an incubator at 24 °C and transferred into 3 l pots arranged in a greenhouse using a completely randomized design. The number of replications per family was from two to four in the season 2005–2006 (average 3.36±0.07) and from seven to eight in the season 2006–2007 (7.40±0.10). The number of full-sibs families for populations SB, BG and AM in the season 2005–2006 was 55, 63 and 70, and in the season 2006–2007 it was 55, 53 and 54, respectively. In total, 685 and 1316 plants were grown in the two experiments. The pots were filled with the sieved soil from original population location (loess, rendzina and Terra Rossa for SB, BG and AM population, respectively). During these experiments, plants received an amount of water as close as possible to the multiyear average rainfall amount recorded by the meteorological service (95, 418 and 595 mm over years 1961–2005 for SB, BG and AM location, respectively), but compensated for higher evaporation rate as compared with natural soil conditions. Evaporation rate was adjusted for ambient temperature. The winter season of 2005–2006 was warmer than the following season and this is reflected in the water amount supplied to the plants. The plants received 188, 328 and 555 mm in the season 2005–2006, and 77, 295 and 419 mm in the season 2006–2007 (SB, BG and AM origin, respectively). Watering was carried out twice a week through drip-irrigation system. The estimates of plant fitness included total number of seeds produced by a plant and total weight of seeds produced by a plant. As spikelets obtained by hand pollination were often smaller than self-pollinated ones, the F1 spikelets used in the 2005–2006 experiment were individually weighted and spikelet weight was used as a covariate. There was no need for individual weighing of F2 spikelets because of low variation in their sizes.
An additional experiment was set up to estimate fitness at the early stage of plant development. In this experiment, seeds of F2 and F3 were arranged in boxes 8 × 8 × 10 cm filled with a soil from original population location as a grid of 4 × 4 seeds (F2) or 6 × 6 seeds (F3). Two replicates were used for each family and the number of germinated seeds in two boxes was pooled to estimate seed viability measured as germination fraction. Water was applied twice simulating two events of heavy rain (15 mm of rainfall) separated by one week. Amount of 10 mm of rainfall is considered a threshold for germination of wild barley (Gutterman, 1993).
Assessment of genetic relatedness
Ten polymorphic and presumably neutral nuclear microsatellite loci (Bmac0030, Bmac0113, Bmac0316, Bmag0222, Bmac0218, EBmac0824, EBmac0403, HVM20, HvOle and WMC1E8, for details, see Volis et al., 2010) were used to estimate the plant genetic relatedness. The loci were amplified with PCR according to Schuelke (2000) with minor modifications, and stained with the fluorescent-labeled M13(-21) primers FAM, NED, PET and VIC. The PCR products were detected and sized by the ABI PRISM 3700 DNA Analyzer at the Hebrew University, Jerusalem, Israel. The data were analyzed using Peak Scanner Software v1.0 (Applied Biosystems, Carlsbad, CA, USA).
In our study, the distances between sampled seeds along transects were the exact one-dimensional coordinates for each sampled plant. Therefore, the pairwise genetic relatedness (kinship) coefficients were obtained for each transect separately. We estimated the kinship coefficients for all pairs of individuals in transects using the statistic of Ritland (1996) as implemented in GENALEX (Peakall and Smouth, 2006). The mean population multilocus kinship coefficients for each distance class were provided with 2SE obtained by jackknifing over transects.
Data analysis
The general linear model of Statistica (StatSoft Inc., 2004) was used to analyze two performance traits, seed number and total seed mass, separately for F1 and F2 hybrids as they were measured in two separate experiments. The model included as terms population, transect nested within population, crossing distance and interactions of crossing distance with population and with transect. Population and crossing distance were treated as fixed effects and transect was treated as a random effect. The F1 data analysis included also initial spikelet weight as a covariate. Before entering this covariate as an effect, homogeneity of the slopes assumption of analysis of covariance was tested for this variable. As an interaction of initial spikelet weight with all other effects was not significant, it was entered into the GLM.
Similar generalized linear model of Statistica (StatSoft Inc., 2004) including all the above factors and their interactions was used to analyze germination percentage. The model assumed Poisson distribution for the errors and the logit link.
To estimate outbreeding depression or heterosis effect, RP was calculated for each generation/family following Agren and Schemske (1993) as RP=(wh−ws)/max(ws, wh) where ws is the fitness of selfed ‘zero-plants’ and wh is the fitness of F1, F2 or F3 progeny of hybrids for crossing distance h. The RP values are bounded by –1 and +1 with positive values indicating higher fitness of hybrids as compared with selfed individuals.
An effect of genetic relatedness and crossing distance on performance of F1, F2 and F3 plants was tested through simple linear and polynomial regression analyses.
Results
Population differences in heterozygosity
In all three populations expected heterozygosity was high, showing that in this species genetic diversity at population level is high in spite of the selfing breeding structure.
Two populations (SB and AM) had low observed heterozygosity (0.078 and 0.008) and high inbreeding coefficient (0.895 and 0.981), consistent with data repeatedly reported in the literature. In contrast, the BG population had an atypically high number of heterozygotes (Ho=0.251 and FIS=0.593) (Table 1).
Effect of genetic relatedness of parents
The kinship coefficients calculated for distance classes ranging 1–400 m indicated spatial genetic structure at a scale of between 1 and 2 m (Table 1), consistent with the results of an explicit analysis of spatial autocorrelation (Volis et al., 2010). Therefore, on average, crosses between plants separated by more than 2 m produced progeny more outbred than crosses between plants within that range.
There was no linear or curvilinear effect of genetic relatedness on performance of either F1 (fecundity), F2 (fecundity and seed viability) or F3 generations (seed viability) (R2 ranged 0.001–0.107, P>0.05) for any population and over all populations.
Effect of crossing distance
Germination of F2, but not F3 seeds was affected by whether their self-pollinated parents came from out-crossed or self-pollinated P plants (Figure 2). The average RP for the F2 generation across all crossing distances (1–400 m for SB and AM populations and 1–100 m for BG population) for germination percentage was −0.051, −0.160, −0.202 (t=3.3, 5.0, 9.2; P=0.017, 0.002, 0.001; one-sample t-test) for SB, BG and AM, respectively, indicating an outbreeding depression effect in all three populations (Figure 2). In the F3 generation, a small outbreeding depression effect was still present in the BG population (RP=−0.053; t=3.2, P=0.017; one-sample t-test), but not in the other two populations (RP=−0.001 and −0.013; t=0.1 and 1.0, P>0.1; one-sample t-test) (Figure 2). There was an effect of crossing distance on germination percentage in F2 but not in F3 (Table 2). In addition, in the F2 generation, effects of population, transect and transect × cross-distance were also significant (Table 2), indicating that outbreeding depression effect differed among the populations and transects (that is, offspring of different mother plants).
RP estimated as differences in seed viability in F2 and F3 plants (±s.e.) that resulted from crosses between plants separated by increasing distance in three populations of wild barley. Positive and negative values denote better and poorer performance, respectively, of the hybrids as compared with the self-pollinated mother plants.
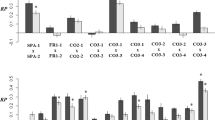
Seed number and total seed mass showed similar responses in the experiments, both being significantly influenced by population and transect with no effect of crossing distance and any interaction in F1. In the F2, the picture was the same except for no effect of population for seed number (Table 2). In the F1, the seed number and seed mass RP for hybrids was −0.093, 0.045, −0.040 (t=6.4, 0.9, 2.0; P=0.001, 0.423, 0.088; one-sample t-test) and −0.098, 0.105, 0.024 (t=5.9, 2.0, 1.2; P=0.001, 0.094, 0.280; one-sample t-test) (SB, BG and AM, respectively), indicating heterosis effect in BG population, outbreeding depression effect in SB population and no clear effect in the AM population (Figure 3). In the F2, the seed number and seed mass RP for hybrids was 0.008, −0.078, 0.027 (t=0.3, 8.3, 2.7; P=0.747, 0.001, 0.029; one-sample t-test) and 0.063, −0.052, −0.003 (t=2.7, 7.5, 0.2; P=0.034, 0.001, 0.852, one-sample t-test) (SB, BG and AM, respectively), showing outbreeding depression in BG population, and no consistent effect in the other two populations (Figure 3).
RP estimated as differences in seed number (left) and total seed mass (right) in F1 and F2 plants (±s.e.) that resulted from crosses between plants separated by increasing distance in three populations of wild barley. Positive and negative values denote better and poorer performance, respectively, of the hybrids as compared with the self-pollinated mother plants.
A regression between the plant RP and the logarithm of crossing distance revealed no simple linear relationship for any fitness component in any of the three populations. A curvilinear relationship between the logarithm of crossing distance and some fitness components was found in the BG and AM populations, indicating a maximum of hybrid vigor (F1) and outbreeding depression (F2) at intermediate distances (Table 3). This relationship, however, became not significant after sequential Bonferroni correction.
There was no difference in variation in either seed number or seed mass between ‘zero-plant’ parent and F2 progeny from particular distance class (Levene's test of variance homogeneity, P>0.05). However, in two populations, AM and especially in the SB population, a range of variation in F2 in many distance classes was higher than in the ‘zero-plant’ parent (Figure 4), showing presence of new recombined genotypes with improved or reduced fitness. In the BG population, the opposite variation pattern was observed, viz wide range of variation in F1 and its reduction in F2.
Discussion
Outbreeding depression or hybrid breakdown, defined as a reduction in fitness of hybrids as compared with progeny derived through self-fertilization (a specific case of outbreding depression for a self-pollinated organism), includes two possible mechanisms. Each mechanism implies an appropriate scale over which the outbreeding depression effect is expected. An ‘ecological’ mechanism (Price and Waser, 1979) is applicable to the cases when genotypes adapted to particular habitats are crossed. The appropriate scale for this mechanism is related to the spatial changes in selection regime. In our study, the linear transects were set randomly in each population location and the environment occupied by a population had no distinct microhabitats or clines of abiotic/biotic effects. The latter was an important criterion for choosing the three populations. The autocorrelation effect was not significant for any distance class, when available nitrogen was used as a proxy to spatial heterogeneity in soil. However, in spite of no indication of spatial heterogeneity, local adaptation and ecological mechanism can not be ruled out because adaptive genetic differentiation in plants can occur at the very fine spatial scale. For example, adaptive microevolution of plant resistance to pathogens was shown at a scale of meters in Plantago lanceolata (Laine, 2006). In this study, the host plants showed higher resistance to sympatric than to allopatric strains of powdery mildew Podoshaera plantaginis. A similar process, viz local adaptation to particular strains of the same pathogen or a combination of several pathogen species, can be invoked to explain the observed pattern of spatial genetic structure and outbreeding depression in wild barley. However, this process is not mutually exclusive with a ‘genetic’ mechanism of outbreeding depression. We explicitly designed our study to test for the ‘genetic’ mechanism, which hypothesizes that combined effects of uniform selection, limited gene flow between and drift within sub-populations lead to rapid fixation of coadapted gene complexes by epistatic interactions, which break up in sub-population crosses (Price and Waser, 1979; Campbell and Waser, 1987). The different coadapted gene combinations can be viewed as ‘adaptive peaks’, which evolved as multiple solutions to the same environmental challenge and not as a response to differential selection caused by environmental heterogeneity (Whitlock et al., 1995; Bieri and Kawecki, 2003). A scale, at which outbreeding depression, that is, breakage of these gene complexes, will occur in hybrids, will be a function of a spatial distance of major gene flow through pollen and seeds (Campbell and Waser, 1987; Epperson, 1989; Heywood, 1991).
The genetic mechanism of outbreeding depression predicts that under the strong effect of drift combined with limited dispersal we expect individuals located in close proximity to be more genetically similar, and therefore to show less outbreeding depression and less heterosis. We found spatial genetic structure at a scale of between 1 and 2 m (Volis et al., 2010 and this study) implying that plants within this distance are genetically closely related producing progeny more inbred than other crosses.
However, we did not find a clear spatial pattern in effect of hybridization. An apparent nonlinear relationship of geographic distance and performance of the cross progeny detected in the two of three populations became not significant after sequential Bonferroni adjustment and plant performance was independent of plant genetic relatedness. This can indicate that a distance of 1 m, the smallest sampling/crossing distance used in this study, exceeds the radius of major seed dispersal and genetic neighborhood in wild barley. Indeed, primary seed dispersal in wild barley (⩾95%), measured experimentally, was estimated to be within 1.2 m of the seed plant (Volis et al., 2010). Thus, a possible explanation for a lack of association between RP of hybrids and either genetic relatedness of parents or crossing distance is that plants separated by as small distance as 1 m represent differentiated gene pools. As these gene pools can partly overlap, plants 1–2 m apart are more genetically similar than those separated by larger distances, but still differ in epistatic interactions. As a result of fine spatial, mosaic-like, genetic structure, magnitude of the difference in epistatic interactions does not change with distance.
There was high variation in plant performances across populations, transects and crossing distances (in decreasing order). On average, in spite of the small in magnitude fitness effects, occasional hybridization (that is, cross-pollination) seems to produce more plants with superior than inferior fitness as compared with self-progeny. However, this heterosis effect is very short lived and disappears or becomes negligible after a single generation of self-pollination. Our results support theoretical predictions that heterosis effects are most evident in the first generation of crossing, while effects of epistasis are revealed in subsequent generations under segregation and recombination of loci (Lynch, 1991). For example, a strong effect of outbreeding depression was observed for the F3 generation of hybrids between two biotypes of Amphicarpea bracteata, a highly selfing annual plant (Parker, 1992). Parker (1992) attributed this effect to intrinsic coadaptation and not to local adaptation because of lack of any association of the biotypes with distinct environments. Interestingly, while most hybrids in this study had low fitness, a single most fit plant was a hybrid individual.
In our study, an outbreeding depression effect was observed in the F2 generation for seed germination ability. However, in the F3 generation this effect disappeared. Thus purging selection, responsible for disappearance of the outbreeding depression effect in the F3, seems to operate at the earliest stage of the life cycle, through viability and germination ability of seeds. Elimination of new maladaptive gene combinations after a few generations of selfing witnesses a strong purging selection in wild barley.
Rapid disappearance of outbreeding effect was also found in a highly inbred perennial herb Anchusa crispa (Quilichini et al., 2001). In this species, outbreeding depression was expressed in both the F1 and F2 generations but not when selfed and outcrossed F2 produced from selfed F1 were compared. The negative outbreeding effect was observed on a highly localized scale between patches of individuals several meters apart. Another similarity between our results and those reported in Quilichini et al. (2001) is that in both cases outbreeding depression was expressed not in seed set but in seed quality evident either during seed germination (this study) or seedling growth (Quilichini et al., 2001).
Interestingly, heterosis in F1 and outbreeding depression in F2 for seed production were observed only in the population with unusually high number of heterozygotes (BG). This fits well a hypothesis that spatially isolated highly inbred genotypes are co-adapted gene complexes that were sieved by selection, while heterozygotes are characteristic for the transient phase of this process, when segregation and purging of maladaptive genotypes have not yet occurred.
There is a certain parallel between our results and observations of Zohary on a fate of spontaneous hybrid swarms of wild two-rowed and cultivated six-rowed barley that are sporadically encountered at edges of barley cultivation and in disturbed contact sites (Zohary, 1964, 1971). These swarms contained parental forms and a diverse range of intermediates and recombinants (up to 5% of the total population). Observations on population dynamics of these swarms revealed dramatic change in their diversity, with typical wild form taking over completely in only 2 to 3 years. It seems to be a general rule that inferiorly fit recombinant genotypes are purged very quickly from the barley population.
In summary, our study provides some insights on evolutionary importance of (i) spatial structure and (ii) occasional cross-pollination, in predominantly selfing species. The commonly observed population structure of divergent multilocus genotypes in selfing plant species was hypothesized to be the outcome of selection for the most fit coadapted sets of genes (Allard, 1975; Allard et al., 1993). Yet, this hypothesis received little empirical support (Hedrick and Holden, 1979; Golenberg, 1987; Keller and Waller, 2002). In our study, we observed a fine-scale spatial structure and a reduction in fitness of recombined genotypes in the first generation of segregation. These findings support the view that a spatial mosaic of various locally abundant genotypes represents not randomly fixed sets of alleles but the best fit coadapted combinations of the latter given sufficient genetic variation in a population for selection to work on. The coadapted gene complexes in wild barley can represent different solutions to either the same environmental problem (‘adaptive peaks’) or to the multiple biotic/abiotic problems at the very fine spatial scale (local microadaptations). A situation can be even more complicated with both mechanisms simultaneously present and operating at the similar spatial scale ⩽2 m at which major seed dispersal occurs (Volis et al., 2010). In contrast, some gene flow through sexual recombination seems to be important in a predominant selfer to prevent accumulation of mildly deleterious mutations (Fritsche and Kaltz, 2000; Lenormand and Otto, 2000; Johansen-Morris and Latta, 2006) and fixation of beneficial alleles in different genetic neighborhoods (Hill and Robertson, 1966; Felsenstein, 1974). In predominant selfers, occasional sexual recombination creates new combinations of alleles among and within loci, of which only a small fraction will persist over generations because of higher fitness than in existing combinations. However, occasional creation of such genotypes may be an important mechanism by which the beneficial mutations are brought together within the same individual (Muller, 1932; Barton and Otto, 2005; Martin et al., 2006; Roze and Barton, 2006). An evolutionary advantage of the latter effect can be especially pronounced under changing environmental conditions. Ronfort and Couvet (1995) showed that population subdivision (which is commonly observed in predominant selfers) creates conditions for evolutionary stable mixed mating system. A degree of outcrossing in predominant selfers may reflect importance of this mechanism in evolution of a particular species. It should be noted that although our findings are in general consistent with the multiple peak adaptive landscape theory of Wright (Wright, 1931) further theoretical developments and modeling of multiple-peak epistasis in a subdivided population incorporating selfing/inbreeding are needed.
References
Agren J, Schemske DW (1993). Outcrossing rate and inbreeding depression in two annual monoecious herbs, Begonia hirsute and B. semiovata. Evolution 47: 125–135.
Allard RW (1975). The mating system and microevolution. Genetics 79: 115–126.
Allard RW, Barbel GR, Clegg MT, Kahler AL (1972). Evidence for coadaptation in Avena barbata. Proc Natl Acad Sci USA 69: 3043–3048.
Allard RW, Garcia P, Saenz-de-Miera LE, Perez de la Vega M (1993). Evolution of multilocus genetic structure in Avena hirtula and Avena barbata. Genetics 135: 1125–1139.
Barton NH, Otto SP (2005). Evolution of recombination due to random drift. Genetics 169: 2353–2370.
Bieri J, Kawecki TJ (2003). Genetic architecture of differences between populations of cowpea weevil (Callosobruchus maculatus) evolved in the same environment. Evolution 57: 274–287.
Bonnin I, Ronfort J, Wozniak F, Olivieri I (2001). Spatial effects and rare outcrossing events in Medicago truncatula (Fabaceae). Mol Ecol 10: 1371–1383.
Brown AHD, Zohary D, Nevo E (1978). Outcrossing rates and heterozygosity in natural populations of Hordeum spontaneum Koch in Israel. Heredity 41: 49–62.
Brown JH, Kodric-Brown A (1977). Turnover rates in insular biogeography: effect of immigration on extinction. Ecology 58: 445–449.
Campbell DR, Waser NM (1987). The evolution of plant mating systems—multilocus simulations of pollen dispersal. Am Natural 129: 593–609.
Campbell DR, Waser NM (2001). Genotype-by-environment interaction and the fitness of plant hybrids in the wild. Evolution 55: 669–676.
Charlesworth B (1990). Mutation-selection balance and the evolutionary advantage of sex and recombination. Genet Res 55: 199–221.
Charlesworth D (2003). Effects of inbreeding on the genetic diversity of populations. Philos Trans R Soc London Ser B-Biol Sci 358: 1051–1070.
Edmands S (2007). Between a rock and a hard place: evaluating the relative risks of inbreeding and outbreeding for conservation and management. Mol Ecol 16: 463–475.
Epperson BK (1989). Spatial patterns of genetic variation within plant populations. In: Brown ADH, Clegg MT, Kahler AL and Weir BS (eds). Plant Population Genetics, Breeding and Genetic Resources. Sinauer: Sunderland, MA.
Felsenstein J (1974). The evolutionary advantage of recombination. Genetics 78: 737–756.
Fenster CB, Galloway LF, Chao L (1997). Epistasis and its consequencies for the evolution of natural populations. Trends Ecol Evol 12: 282–286.
Fisher RA (1918). The correlation between relatives on the supposition of Mendelian inheritance. Philos Trans R Soc Edinburgh 3: 399–433.
Fritsche F, Kaltz O (2000). Is the Prunella (Lamiaceae) hybrid zone structured by an environmental gradient? Evidence from a reciprocal transplant experiment. Am J Botany 87: 995–1003.
Garris AJ, McCouch SR, Kresovich S (2003). Population structure and its effect on haplotype diversity and linkage disequilibrium surrounding the xa5 locus of rice (Oryza sativa L. Genetics 165: 759–769.
Golenberg EM (1987). Estimation of gene flow and genetic neighborhood size by indirect methods in a selfing annual, Triticum dicoccoides. Evolution 41: 1326–1334.
Gutterman Y (1993). Seed Germination in Desert Plants. Springer Verlag: Berlin.
Hamblin MT, Mitchell SE, White GM, Gallego J, Kukatla R, Wing RA et al. (2004). Comparative population genetics of the panicoid grasses: sequence polymorphism, linkage disequilibrium and selection in a diverse sample of Sorghum bicolor. Genetics 167: 471–483.
Hamrick JL, Holden LR (1979). Influence of microhabitat heterogeneity on gene frequency distribution and gametic phase disequilibrium in Avena barbata. Evolution 33: 521–533.
Harlan RJ, Zohary D (1966). Distribution of wild wheats and barley. Science 153: 1074–1080.
Hedrick PW, Holden L (1979). Hitch-hiking: an alternative to coadaptation for the barley and slender wild oat examples. Heredity 43: 79–86.
Heywood JS (1991). Spatial analysis of genetic variation in plant populations. Ann Rev Ecol Syst 22: 335–355.
Hill WG, Robertson A (1966). The effect of linkage on limits to artificial selection. Genet Res 8: 269–294.
Huang Q, Beharav A, Kirzhner V, E N (2002). Mosaic microecological differential stress causes adaptive microsatellite divergence in wild barley, Hordeum spontaneum, at Neve Yaar, Israel. Genome 45: 1216–1229.
Hufford KM, Mazer SJ (2003). Plant ecotypes: genetic differentiation in the age of ecological restoration. Trends Ecol Evol 18: 147–155.
Johansen-Morris AD, Latta RG (2006). Fitness consequences of hybridization between ecotypes of Avena barbata: hybrid breakdown, hybrid vigor, and transgressive segregation. Evolution 60: 1585–1595.
Keller LF, Waller DM (2002). Inbreeding effects in wild populations. Trends Ecol Evol 17: 230–241.
Kondrashov AS (1988). Deleterious mutations and the evolution of sexual reproduction. Nature 336: 435–440.
Laine A-L (2006). Evolution of host resistance: looking for coevolutionary hotspots at small spatial scales. Proc R Soc B-Biol Sci 273: 267–273.
Latta RG, MacKenzie JL, Vats A, Schoen DJ (2004). Divergence and variation of quantitative traits between allozyme genotypes of Avena barbata from contrasting habitats. J Ecol 92: 57–71.
Lechowicz MJ, Bell G (1991). The ecology and genetics of fitness in forest plants. 2. Microspatial heterogeneity of the edaphic environment. J Ecol 79: 687–696.
Lenormand T, Otto SP (2000). The evolution of recombination in a heterogeneous environment. Genetics 156: 423–438.
Lonn M (1993). Genetic structure and allozyme-microhabitat associations in Bromus hordeaceus. Oikos 68: 99–106.
Lynch JM (1991). The genetic interpretation of inbreeding depression and outbreeding depression. Evolution 45: 622–629.
Martin G, Otto SP, Lenormand T (2006). Selection for recombination in structured populations. Genetics 172: 593–609.
Miller RE, Hoef JMV, Fowler NL (1995). Spatial heterogeneity in eight central Texas grasslands. J Ecol 83: 919–928.
Morrell PL, Lundy KE, Clegg MT (2003). Distinct geographic patterns of genetic diversity are maintained in wild barley (Hordeum vulgare ssp. spontaneum) despite migration. Proc Natl Acad Sci USA 100: 10812–10817.
Morrell PL, Toleno DM, Lundy KE, Clegg MT (2005). Low levels of linkage disequilibrium in wild barley (Hordeum vulgare ssp. spontaneum) despite high rates of self-fertilization. Proc Natl Acad Sci USA 102: 2442–2447.
Muller HJ (1932). Some genetic aspects of sex. Am Natural 66: 118–138.
Mulvaney RL (1996). Nitrogen—inorganic forms. In: Sparks DL (ed). Methods of Soil Analysis. Part 3. Chemical Methods. Soil Science Society of America: Madison, WI. pp 1123–1184.
Nevo E, Beharav A, Meyer RC, Hackett CA, Forster BP, Russell JR et al. (2005). Genomic microsatellite adaptive divergence of wild barley by microclimatic stress in ‘Evolution Canyon’, Israel. Biol J Linnean Soc 84: 205–224.
Nevo E, Beiles A, Kaplan D, Golenberg EM (1986). Natural selection of allozyme polymorphisms: a microsite test revealing ecological genetic differentiation in wild barley. Evolution 40: 13–20.
Nordborg M (2000). Linkage disequilibrium, gene trees and selfing: an ancestral recombination graph with partial self-fertilization. Genetics 154: 923–929.
Nordborg M, Borevitz JO, Bergelson J, Berry CC, Chory J, Hagenblad J et al. (2002). The extent of linkage disequilibrium in Arabidopsis thaliana. Nat Genet 30: 190–193.
Parker MA (1992). Outbreeding depression in a selfing annual. Evolution 46: 837–841.
Peakall R, Smouse PE (2006). GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol 6: 288–295.
Price MV, Waser NM (1979). Pollen dispersal and optimal outcrossing in Delphinium nelsoni. Nature 277: 294–297.
Quilichini A, Debussche M, Thompson JD (2001). Evidence for local outbreeding depression in the Mediterranean island endemic Anchusa crispa Viv. (Boraginaceae). Heredity 87: 190–197.
Ramakrishnan AP, Meyer SE, Fairbanks DJ, Coleman CE (2006). Ecological significance of microsatellite variation in western North American populations of Bromus tectorum. Plant Species Biol 21: 61–73.
Ritland K (1996). Estimators for pairwise relatedness and individual inbreeding coefficients. Genet Res 67: 175–185.
Ronfort J, Couvet D (1995). A stochastic model of selection on selfing rates in structured populations. Genet Res 65: 209–222.
Roze D, Barton NH (2006). The Hill-Robertson effect and the evolution of recombination. Genetics 173: 1793–1811.
Schlesinger WH, Raikes JA, Hartley AE, Cross AE (1996). On the spatial pattern of soil nutrients in desert ecosystems. Ecology 77: 364–374.
Schuelke M (2000). An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol 18: 233–234.
Siol M, Bonnin I, Olivieri I, Prosperi JM, Ronfort J (2007). Effective population size associated with self-fertilization: lessons from temporal changes in allele frequencies in the selfing annual Medicago truncatula. J Evol Biol 20: 2349–2360.
Siol M, Prosperi J, Bonnin I, Ronfort J (2008). How multilocus genotypic pattern helps to understand the history of selfing populations: a case study in Medicago truncatula. Heredity 100: 517–525.
StatSoft Inc (2004). STATISTICA (data analysis software system), version 7, www.statsoft.com.
Tallmon DA, Luikart G, Waples RS (2004). The alluring simplicity and complex reality of genetic rescue. Trends Ecol Evol 19: 489–496.
Verhoeven KJF, Vanhala TK, Biere A, Nevo E, Van Damme JMM (2004). The genetic basis of adaptive population differentiation: a quantitative trait locus analysis of fitness traits in two wild barley populations from contrasting habitats. Evolution 58: 270–283.
Volis S, Mendlinger S, Ward D (2002). Differentiation in populations of Hordeum spontaneum along a gradient of environmental productivity and predictability: life history and local adaptation. Biol J Linnean Soc 77: 479–490.
Volis S, Olsvig-Whittaker L, Anikster Y, Mendlinger S (2004a). The influence of space in genetic-environmental relationships when environmental heterogeneity and seed dispersal occurs at similar scale. Am Natural 163: 312–327.
Volis S, Verhoeven K, Mendlinger S, Ward D (2004b). Phenotypic selection and regulation of reproduction in different environments in wild barley. J Evol Biol 17: 1121–1131.
Volis S, Zaretsky M, Shulgina I (2010). Fine-scale spatial genetic structure in a predominantly selfing plant: role of seed and pollen dispersal. Heredity 105: 384–393..
Wagner DB, Allard RW (1991). Pollen migration in predominantly self-fertilizing plants: barley. J Hered 82: 302–304.
Whitlock MC, Phillips PC, Moore FBG, Tonsor SJ (1995). Multiple fitness peaks and epistasis. Ann Rev Ecol Syst 26: 601–629.
Williams CF (1994). Genetic consequences of seed dispersal in three sympatric forest herbs. II. Microspatial genetic structure within populations. Evolution 48: 1959–1972.
Wolf JD, Brodie ED, Wade MJ (eds) (2000). Epistasis and the Evolutionary Process. Oxford University Press: Oxford.
Wright S (1931). Evolution in Mendelian populations. Genetics 16: 97–159.
Zhu YL, Song QJ, Hyten DL, Van Tassell CP, Matukumalli LK, Grimm DR et al. (2003). Single-nucleotide polymorphisms in soybean. Genetics 163: 1123–1134.
Zohary D (1964). Barley genetics I. Proceedings of 1st International Barley Genetics Symposium, Wageningen 1963. Pudoc: Wageningen, The Netherlands, 27–31.
Zohary D (1971). The fate of natural “hybrid swarms” between Hordeum spontaneum and H. vulgare. In: Barley Genetics II. Proceedings of 2nd International Barley Genetics Symposium. pp 63–64.
Acknowledgements
We thank Gil Bohrer, Christian Parisod, Frank Sorensen, Koen Verhoeven, Allan Witztum and three anonymous reviewers for helpful comments on an early version of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Volis, S., Shulgina, I., Zaretsky, M. et al. Epistasis in natural populations of a predominantly selfing plant. Heredity 106, 300–309 (2011). https://doi.org/10.1038/hdy.2010.79
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hdy.2010.79