Abstract
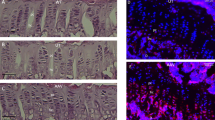
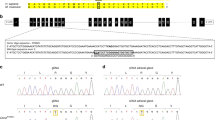
The treatment of severe forms of 21-hydroxylase deficiency (21OHD) remains unsatisfactory in many respects. As a monogenic disease caused by loss-of-function mutations, 21OHD is a potential candidate for a gene therapy (GT) approach. The first step of GT is to demonstrate positive effects of the therapeutic vector in the Cyp21−/− mouse model. Thus, we tested the adrenal tropism of an AAVrh10-CAG-GFP vector (‘GFP vector’) then attempted to correct the phenotypic and biochemical alterations in Cyp21−/− mice using an AAVrh10-CAG-humanCYP21A2-HA vector (‘CYP21 vector’). Cyp21−/− mice had decreased body mass, high progesterone (4 ×), impaired stress response, increased adrenal expression of genes involved in steroidogenesis or ACTH signaling. Following injection of the GFP vector, Cyp21−/− mice showed abundant GFP expression in the adrenal cortex. Intravenous injection of the therapeutic CYP21 vector allowed 21OH expression in adrenal tissue, resulting in increased body weight and near normalization of urinary progesterone for more than 15 weeks, improved response to stress and restoration of near-normal expression of (several important genes) in the adrenal cortex. The adrenal tropism of AAVrh10 and the persistent correction of phenotypic and biochemical traits in Cyp21−/− mice pave a first step on the way to GT of 21OHD in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gmyrek GA, New MI, Sosa RE, Poppas DP . Bilateral laparoscopic adrenalectomy as a treatment for classic congenital adrenal hyperplasia attributable to 21-hydroxylase deficiency. Pediatrics 2002; 109: E28.
Bruining H, Bootsma AH, Koper JW, Bonjer J, de Jong FH, Lamberts SWJ . Fertility and body composition after laparoscopic bilateral adrenalectomy in a 30-year-old female with congenital adrenal hyperplasia. J Clin Endocrinol Metab 2001; 86: 482–484.
Loechner KJ, McLaughlin JT, Calikoglu AS . Alternative strategies for the treatment of classical congenital adrenal hyperplasia: pitfalls and promises. Int J Pediatr Endocrinol 2010; 2010: 1–10.
Gastaud F, Bouvattier C, Duranteau L, Brauner R, Thibaud E, Kutten F et al. Impaired sexual and reproductive outcomes in women with classical forms of congenital adrenal hyperplasia. J Clin Endocrinol Metab 2007; 92: 1391–1396.
Riepe FG, Tatzel S, Sippell WG, Pleiss J, Krone N . Congenital adrenal hyperplasia: the molecular basis of 21-hydroxylase deficiency in H-2(aw18) mice. Endocrinology 2005; 146: 2563–2574.
Tajima T, Ma XM, Bornstein SR, Aguilera G . Prenatal dexamethasone treatment does not prevent alterations of the hypothalamic pituitary adrenal axis in steroid 21-hydroxylase deficient mice. Endocrinology 1999; 140: 3354–3362.
Bornstein SR, Tajima T, Eisenhofer G, Haidan A, Aguilera G . Adrenomedullary function is severely impaired in 21-hydroxylase-deficient mice. FASEB J 1999; 13: 1185–1194.
Tajima T, Okada T, Ma XM, Ramsey W, Bornstein S, Aguilera G . Restoration of adrenal steroidogenesis by adenovirus-mediated transfer of human cytochromeP450 21-hydroxylase into the adrenal gland of21-hydroxylase-deficient mice. Gene Therapy 1999; 6: 1898–1903.
Macapagal MC, Slowinska BS, Nimkarn S, De BP, Licholai T, MI et al Gene therapy of 21-hydroxylase deficient mice utilizing an adeno-associated virus vector. Abstract the Endocrine Society’s 84th Annual Meeting, San Francisco, 2002, pp 1–503.
Naiki Y, Miyado M, Horikawa R, Katsumata N, Onodera M, Pang S et al. Extra-adrenal induction of Cyp21a1 ameliorates systemic steroid metabolism in a mouse model of congenital adrenal hyperplasia. Endocr J 2016; 63: 897–904.
Alexander IE, Russell DW . The potential of AAV-mediated gene targeting for gene and cell therapy applications. Curr Stem Cell Rep 2015; 1: 16–22.
Thwaite R, Pagès G, Chillón M, Bosch A . AAVrh.10 immunogenicity in mice and humans. Relevance of antibody cross-reactivity in human gene therapy. Gene Therapy 2015; 22: 196–201.
Vance MA, Mitchell A, Samulski RJ . AAV Biology, Infectivity and Therapeutic Use from Bench to Clinic Gene Therapy—Principles and Challenges, Chapter 5 InTech, 2015, pp 119–143.
Parker KL, Chaplin DD, Wong M, Seidman JG, Smith JA, Schimmer BP . Expression of murine 21-hydroxylase in mouse adrenal glands and in transfected Y1 adrenocortical tumor cells. Proc Natl Acad Sci USA 1985; 82: 7860–7864.
Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 1998; 20: 1093–1102.
Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W et al. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci 2005; 25: 6243–6250.
Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 2000; 24: 410–414.
Bland ML, Jamieson CAM, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF et al. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA 2000; 97: 14488–14493.
Sapolsky RM, Romero LM, Munck AU . How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000; 21: 55–89.
Acknowledgements
We thank the International Fund for research on Congenital Adrenal Hyperplasia (IFCAH) and the French Muscular Dystrophy Association (AFM-Telethon) for supporting the project. We thank P Aubourg for his help for the choice of vector, K Cambon (MIRCen) for sharing her expertise on stress studies in mice, C Gomez for providing antibody against aldosterone synthase and D Aubert for the production of the sham plasmid. We also thank T Haudebourg, C Sevin, C Bouvattier, N Lefoulon, L Breton and S Guidoux-Boralevi for their technical contribution at the beginning of the project years before its complete re-orientation in 2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Perdomini, M., Dos Santos, C., Goumeaux, C. et al. An AAVrh10-CAG-CYP21-HA vector allows persistent correction of 21-hydroxylase deficiency in a Cyp21−/− mouse model. Gene Ther 24, 275–281 (2017). https://doi.org/10.1038/gt.2017.10
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.10
This article is cited by
-
Enhancing CAR-T cells: unleashing lasting impact potential with phytohemagglutinin activation in in vivo leukemia model
Cancer Gene Therapy (2024)
-
Management challenges and therapeutic advances in congenital adrenal hyperplasia
Nature Reviews Endocrinology (2022)
-
Novel treatments for congenital adrenal hyperplasia
Reviews in Endocrine and Metabolic Disorders (2022)