Abstract
Vaccinia virus (VV) has many attractive characteristics as a potential cancer therapeutic. There are several strains of VV. The nonvaccine strain Western Reserve VV with deletion of both the thymidine kinase and the viral growth factor genes (known as WRDD) has been reported as the most potent tumor-targeted oncolytic VV. Other strains, such as the European vaccine Lister strain, are largely untested. This study evaluated the antitumor potency and biodistribution of different VV strains using in vitro and in vivo models of cancer. Lister strain virus with thymidine kinase gene deletion (VVΔTK) demonstrated superior antitumor potency and cancer-selective replication in vitro and in vivo, compared with WRDD, especially in human cancer cell lines and immune-competent hosts. Further investigation of functional mechanisms revealed that Lister VVΔTK presented favorable viral biodistribution within the tumors, with lower levels of proinflammatory cytokines compared with WRDD, suggesting that Lister strain may induce a diminished host inflammatory response. This study indicates that the Lister strain VVΔTK may be a particularly promising VV strain for the development of the next generation of tumor-targeted oncolytic therapeutics.
Similar content being viewed by others
Introduction
Oncolytic viruses are attractive agents for the treatment of cancers that are resistant to the conventional therapies.1 A first generation replication-selective oncolytic adenovirus with E1B55K deletion has been approved as the world's first oncolytic virus for head and neck cancer therapy.2 Although safety profiles are encouraging, the therapeutic outcomes of clinical trials of viral monotherapy have been disappointing.
The potency of oncolytic adenovirus is limited by its dependence on a specific receptor for entry into the host cell, which can be altered during malignant transformation.3 Vaccinia is an alternative virus with many superior characteristics,4 including rapid replication and cytolysis, wide tumor tropism, nonintegrativewith host DNA, efficient spread to tumors, large cloning capacity, well-defined molecular biology and established treatments for uncontrolled infections. Furthermore, the hypoxic microenvironment commonly found in solid tumors is detrimental to the replication and efficacy of many types of oncolytic viruses,5, 6, 7 but not to vaccinia virus.8 A number of strains of vaccinia virus were used around the world as part of the smallpox eradication program in the 1950 s.9 The use of vaccine-derived strains in cancer therapy has the major benefit that they have been used extensively in human populations, and hence have proven safety. Certain strains have demonstrated tumor selectivity in vitro, whereas other strains have demonstrated no inherent cancer selectivity and no replicative capacity in mammalian cells.4
A comparison of the antitumor potency of different vaccinia strains concluded that non-vaccine Western serve (WR) strain was the most cytotoxic.10 However, this study compared wild-type viruses that are known from clinical trials to be well tolerated, but exhibit relatively poor oncolytic efficacy.4 Furthermore in vitro potency was only compared by using a single method of viral replication assay, and in two human cancer cell lines, A2780 (ovarian) and HCT116 (colorectal). A WR vaccinia virus mutant WRDD, with deletions of both thymidine kinase (TK) and vaccinia growth factor genes, has been shown to exhibit enhanced tumor selectivity and reduced toxicity.11 This improved safety did not compromise antitumor potency; with comparable in vitro cytotoxicity of WRDD compared to the wild-type virus, and superior potency compared to the most commonly used vaccinia strain in clinical trials, Wyeth TK-deleted strain. However, biodistribution studies have demonstrated significant WR viral titers in the ovary and to lesser extent bone marrow,11 raising the prospect of infertility and myelo-suppression following WR treatment.
Recently the UK smallpox vaccine strain, Lister, has been demonstrated to have a superior antitumor potency compared with adenovirus (Ad5) in pancreatic and head and neck cancer cell lines in vitro and in vivo.12, 13 Lister VV has also demonstrated significant cytotoxicity in other types of tumors, such as colorectal, gastric, mesothelioma, lung, thyroid and breast cancer.14 The Lister strain was previously thought to have limited tumor selectivity in vitro.4 Favorable biodistribution of Lister strain has also been demonstrated; with no virus detectable in ovary, spleen or brain tissues following IV injection.12 Moreover, vaccinia viruses express several immunomodulators to evade the host anti-viral immune response,15 such as interferon decoy receptors, inhibitors of Toll-like receptor signaling and NF-κB pathway and chemokine inhibitors. It has been demonstrated that Lister strain encodes more genes involved in immuno-evasion than WR strain such as A53R (a soluble tumor-necrosis factor receptor), and Lister 35 kDa (a viral CC chemokine inhibitor).16 We hypothesized that Lister strain might represent a safer and more potent virus for development of cancer therapeutics compared with the dominant research strain, especially in the immune-competent host.
In this study we have compared the in vitro and in vivo efficacy and biodistribution of tumor-selective WR virus (WRDD) with a tumor-selective mutant of the Lister European smallpox vaccine strain (VVΔTK or VVL15). The nature of the host immune response to these viruses was also examined.
Results
Comparison of the cytotoxicity and replication of vaccinia virus mutants in vitro
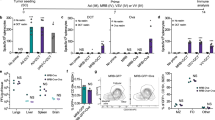
The cytotoxicity of tumor-selective vaccinia mutants of the Lister and WR strains with single thymidine kinase deletions (WRLuc (WR strain) and VVL15 (Lister strain with TK deletion)) and a double-deleted WR with both TK and vaccinia growth factor (VGF) deletions (WRDD) were compared in a large number of human and murine cancer cell lines from a variety of organs (Figures 1a, c and d). The two human cancer cell lines, A2780 and HCT116, used to demonstrate superior WR strain cytotoxicity over other vaccinia strains as published previously,10 demonstrated greater antitumor potency and viral replication with WRDD virus compared with VVL15 (Figures 1a and b). However, WRDD showed inferior cytotoxicity to VVL15 in 10 of 11 other human cancer cell lines tested (Figure 1c) and there was equivalence between VVL15 and WRDD in the human gastric cancer cell line MKN45. All five of the murine cancer cell lines showed greater cytotoxicity when exposed to WRDD compared with VVL15 treatment (Figure 1d).
Cytotoxicity and viral replication of Western Reserve and Lister strain vaccinia viruses in vitro. Cytotoxicity of Lister and WR vaccinia strain mutants in HCT116 colorectal and ovarian A2780 human cancer cell lines. Comparison of mean EC50 values±s.e.m. generated from sextuplicate MTS cell proliferation assays 6 days after infection; 1000 cells per well were infected with different VV at a series of MOI (highest MOI of 1000 pfu cell−1). Statistical analysis was performed using a one-way and two-way analysis of variance test for cytotoxicity and viral replication data respectively; *P<0.05, **P<0.01 and ***P<0.001, ns indicating no statistical significance. (a) Viral replication of Lister and WR vaccinia virus mutants in A2780 and HCT116 cancer cell lines. Burst assays were infected with 1 pfu cell−1 of virus (2 × 105 cells per well). Cell lysates were harvested at 24 h intervals typically up to 96 h. Mean viral replication±s.e.m. was determined by TCID50 assay on CV1 cells (b). Cytotoxicity of Lister and WR vaccinia strain mutants in human cancer cell lines (c) and murine cancer cell lines (d) from a variety of organs were used.
WRLuc showed a more mixed picture compared with WRDD, with superior potency (compared with VVL15 and WRDD) in two mouse cancer cell lines, and 6 out of the 14 human cancer cell lines. Subsequent animal testing with intratumorally delivered WRLuc vaccinia virus (1 × 107 PFU) in nude mice demonstrated significant toxicity resulting in animal death (data not shown). Given this apparent in vivo toxicity, and published evidence of equivalent potency of wild-type compared to double-deleted WR vaccinia against cancer cells in vitro and poor mouse survival in nude and immunocompetent mice following intraperitoneal delivery of wild-type and TK-deleted virus, WRLuc was not investigated further.10, 11
Replication of VV in cancer cell lines was quantified by TCID50 assay (Figures 1b, 2a and b). The viral replication in the tested cancer cell line was consistent with the cytotoxicity.
Viral replication of Lister and WR vaccinia viruses in human and murine cancer cell lines. Burst assays were infected with 1 pfu cell−1 of virus (2 × 105 cells per well). Cell lysates were harvested at 24-h intervals typically up to 96 h. A variety of human (a) and murine (b) cancer cell lines from a variety of organs were used. Mean viral replication±s.e.m. was determined by TCID50 assay on CV1 cells.
Tumor selectivity was demonstrated in vitro by comparing viral replication in cancer cells to replication in nonmalignant normal human bronchial epithelial cells for the two viruses. Supplementary Figure S1 illustrates superior tumor-selective replication (values above Y=1) of Lister strain compared with WR strain with a greater VVL15 replication demonstrated in tumor cells than in nonmalignant normal human bronchial epithelial cells.
Comparison of the antitumor potency of vaccinia virus mutants in vivo
Intratumoral treatment of nude and immunocompetent mice bearing human and murine tumors using VVL15 and WRDD viruses was investigated. For the human cancer cell lines, FaDu (Figure 3a) and PT45 (Figure 3b) grown in nude mice, reduced tumor progression was observed following injection of flank tumors with VVL15 compared with WRDD or PBS (three injections of 1 × 107 PFU every other day). Furthermore the improved animal survival was demonstrated in the FaDu model following five injected doses of VVL15 (data not shown). The in vivo antitumor efficacy of the two viruses in nude mice is consistent with the in vitro cytotoxicity and viral replication data.
Comparison of the antitumor potency of Lister and WR vaccinia virus in nude mouse cancer models. Tumor cells were seeded by subcutaneous injection into the right flank of nude mice. When tumors reached 4–5 mm in diameter, 10 mice each received IT injections of PBS, WRDD or VVL15 on days 1, 3 and 5 (3 × 1 × 107 PFU). Tumors were measured twice weekly. Antitumor efficacy of VVL15 and WRDD was compared in the human HNSSC model FaDu in BALB/c nude mice (a), human pancreatic carcinoma model PT45 in ICRF nude mice (b) and murine colorectal carcinoma model CMT93 ICRF nude mice (c).
A murine cancer cell line (CMT93) was used to establish flank tumors in nude mice. Treatment with intratumoral WRDD (three injections of 1 × 107 PFU every other day) resulted in reduced tumor progression and improved animal survival compared with VVL15 (Figure 3c), consistent with the superior cytotoxicity and viral replication demonstrated with WR-derived virus in the CMT93 cell line in vitro.
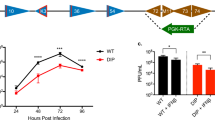
The antitumor potency was further evaluated in immunocompetent mice bearing murine colorectal flank tumors (CMT93 and CT26). Strikingly in the presence of an intact immune system the superior antitumor efficacy by WRDD seen in nude mice was absent, and instead VVL15-treated (3 × 1 × 108 PFU) mice exhibited reduced tumor progression and improved animal survival (Figures 4a and c). This effect was enhanced in the CMT93 model when the dose of virus was increased to five doses at 1 × 108 PFU (Figure 4b).
Comparison of the antitumor potency of Lister and WR vaccinia virus in immunocompetent mouse cancer models. Tumor cells were seeded by subcutaneous injection into the right flank of immunocompetent mice. When tumors reached 4–5 mm in diameter, 10 mice each received IT injections of PBS, WRDD or VVL15 on days 1, 3 and 5 (3 × 1 × 108 PFU) or days 1–5 (5 × 1 × 108 PFU). Antitumor efficacy of VVL15 and WRDD was compared in the CMT93 following the three and five day injection regimen (a,b) and CT26 (c) colorectal carcinoma models. Mean tumor sizes±s.e.m. are displayed until the death of the first mouse in each group. Statistical analysis of tumor sizes at stated time points was performed by using a two-way analysis of variance test. *P<0.05, **P<0.01 and ***P<0.001,ns indicating no statistical significance.
Comparison of viral replication of vaccinia viruses in vivo
In view of the interesting differences in in vivo efficacy, the pattern of replication of the two viruses after local intratumoral administration was investigated. Virus (three doses of 1 × 107 PFU) was injected into nude mice bearing flank tumors of murine colorectal (CMT93) or human pancreatic (PT45) cells. The same experiment was also performed in immunocompetent mice bearing syngeneic colorectal (CMT93) tumors by using three doses of 1 × 108 PFU. Virus in harvested tumors was quantified using quantitative real-time PCR (qRT-PCR) (and TCID50 in Supplementary Figures S2 and S3).
In the nude mouse CMT93 model, titers of WRDD virus were much higher than VVL15 virus (Figure 5a and Supplementary Figure S2). These findings corroborated in vitro cytotoxicity and viral replication assays, where WRDD demonstrated superior antitumor potency in this cell line (Figures 1d and 2b). Furthermore, higher viral titers of WRDD compared with VVL15 translated into reduced tumor progression and improved the animal survival in efficacy studies following intratumoral virus administration to CMT93 flank tumors in nude mice (Figure 3c).
Lister and WR vaccinia virus quantification in harvested murine flank tumors following IT treatment. Tumor cells were seeded by subcutaneous injection into the right flank of mice. When tumors reached 4–5 mm in diameter, 12 mice each received IT injections of PBS, WRDD or VVL15 on days 1, 3 and 5 (3 × 1 × 107 PFU and 3 × 1 × 108 PFU for nude and immunocompetent mice respectively). On specified days mice from each group were sacrificed and tumors were harvested and frozen. Samples were then homogenized and repeatedly freeze-thawed. Lysates underwent DNA extraction. Mean viral genome copy numbers±s.e.m. were determined by qRT-PCR by using the vaccinia late gene (VLTF-1). Virus genome copy number in harvested CMT93 tumors from ICRF nude mice (a) and C57BL/6 immunocompetent mice (b) was compared. Virus genome copy number in harvested PT45 tumors from ICRF nude mice was also established (c). *P<0.05, **P<0.01 and ***P<0.001.
In the immunocompetent CMT93 model, the pattern was reversed with higher levels of VVL15 found compared with WRDD (Figure 5b). The extent of vaccinia viral replication within tumor tissue following intratumoral injection was further evaluated in a model of human cancer. Nude mice bearing pancreatic PT45 flank tumors were injected with virus. Tumors harvested from mice treated with VVL15 demonstrated a higher vaccinia recovery compared to those treated with WRDD (Figure 5c and Supplementary Figure S3). These findings provided additional evidence for a reduced Lister VV clearance from host tumors, resulting in a diminished tumor progression and an improved animal survival (Figure 3b). The contrast between the antitumor potency and intratumoral viral replication of the WR and Lister strain viruses in the nude murine and human models is suggestive of a host species tropism; with Lister strain demonstrating a higher relative human tropism.
Comparison of the biodistribution of vaccinia virus in mouse models of solid tumors in nude and immunocompetent mice after systemic delivery
WR virus, both in wild-type and tumor-targeted double-deleted forms, has been found at significant titers in the ovaries and, to a lesser extent, bone marrow following IP treatment of nude mice bearing MC38 murine colon flank tumors.11 Wild-type Lister strain VV has demonstrated in vivo tumor selectivity as determined by immunohistochemical analysis of harvested organs in nude mice bearing SUIT2 flank tumors following IV viral treatment.12 Comparison was made of the biodistribution of VVL15 and WRDD following a single IV injection in both nude and immunocompetent mice bearing CMT93 murine colorectal flank tumors (1 × 107 and 2 × 108 PFU respectively) by using TCID50, qRT-PCR and immunohistochemical staining to quantify virus from harvested tumors and organs.
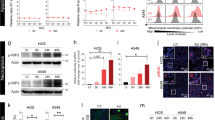
In the nude mouse model, higher titers of WRDD virus were found within tumor tissues across all time points (days 2, 6, 8 and 12), compared with VVL15 (Figure 6a). Similar high levels of WRDD were also found in ovarian tissue, while there were comparatively low levels of virus in ovarian tissue following VVL15 treatment (Figure 6b and Supplementary Figure S4). Analysis of other organs revealed more similar levels of WR and Lister viruses; however, there were statistically significant higher titers of the WRDD virus in liver, spleen, brain and bone marrow compared with VVL15 (Table 1). This suggests that although WRDD virus was able to replicate better in the tumors in nude mice, it was also better able to replicate in several off-target sites and hence was less tumor-selective than VVL15. These findings were consistent across biodistribution analysis methodologies, but best demonstrated by qRT-PCR, which proved to be a more sensitive technique, being able to detect the virus at much lower titers than TCID50 and immunohistochemistry.
Lister and WR biodistribution of vaccinia virus in mice following systemic treatment. Tumor cells were seeded by subcutaneous injection into the right flank of mice. When tumors reached 4–5 mm in diameter, 12 mice each received a single IV injection of PBS, WRDD or VVL15. On selected days four mice from each group were sacrificed and tumors/organs/serum were harvested and frozen. Non-serum samples were then homogenized and repeatedly freeze-thawed. Lysates underwent DNA extraction. Mean viral genome copy numbers±s.e.m. were determined by qRT-PCR using the vaccinia late gene (VLTF-1). By using the CMT93 model, ICRF nude mice were injected with 1 × 107 PFU of vaccinia virus and virus quantified within tumors (a) and ovaries (b) at stated time points. Further assessment in a CMT93 model in immunocompetent C57BL/6 mice following single IV 2 × 108 PFU virus treatment (c) and in a human PT45 nude mouse model (d). Statistical analysis of viral titers at stated time points was performed by using a two-way analysis of variance test; *P<0.05 and **P<0.01 and ***P<0.001, ns indicating no statistical significance.
A similar study was performed by using immunocompetent mice. Much higher levels of virus were found in tumor tissues of mice treated with VVL15 compared with those treated with WRDD (Figure 6c). Therefore, analogous to the findings in the CMT93 efficacy studies, the presence of an intact immune system reverses WRDD superiority to VVL15, suggesting a relative immunotolerance of the host to the Lister strain virus. As in the nude mouse model, high levels of WRDD virus, but not VVL15, were demonstrated in ovarian tissue and to a lesser extent in bone marrow, with equivalent levels of viruses in other organs (Supplementary Table 1).
Biodistribution was studied in nude mice bearing human pancreatic PT45 flank tumors following IV WRDD and VVL15 (1 × 1 × 107 PFU) injection, and very-high levels of Lister virus were found within the tumors, with negligible levels of WR virus (Figure 6d). This suggests VVL15 has high tumor selectivity and ability to replicate in human cancer cells; supported by in vitro viral replication and cytotoxicity assay data, as well as in vivo efficacy for this cancer cell line.
The host immune response to Western Reserve and Lister strain vaccinia virus
The reduced tumor viral clearance and superior antitumor potency of the Lister strain virus, compared with the WR strain virus in immunocompetent models is suggestive of immunologically related differences between the two virus strains. To characterize these potential differences, investigation of the profile of multiple cytokines in murine serum samples following IT delivery of virus to CMT93 flank tumors in immunocompetent mice was undertaken.
Higher levels of proinflammatory cytokines (IL6, IL1β, IFNϒ, IL12 and IL8) were found in the serum of animals treated with WRDD compared with VVL15; in contrast, IL-10, which is considered an anti-inflammatory cytokine was found at higher concentration in animals treated with VVIL15 (Figure 7). This is highly suggestive of WR infections inducing a more potent host antiviral response, with resultant increased viral clearance and hence reduced antitumor potency in immunocompetent in vivo models.
The host cytokine response to Western Reserve and Lister strain vaccinia virus IT treatment. CMT93 cells were seeded by subcutaneous injection into the right flank of C57BL/6 mice. When tumors reached 4-5mm in diameter, nine mice each received IT injections of PBS, WRDD or VVL15 on days 1, 3 and 5. At days 5, 10 and 15, three animals from each group were sacrificed and the serum was sampled. Multiple cytokines were analyzed by ELISA. Statistical analysis was performed using a two-way analysis of variance test; *P<0.05, **P<0.01 and ***P<0.001, ns indicating no statistical significance.
Discussion
In this study, we have demonstrated that a tumor-selective Lister strain virus (VVL15) is more potent than a tumor-selective mutant of the dominant research WR strain (WRDD) in human cancer cell lines in vitro. The WR strain showed greater murine tropism in vitro, in keeping with its development through repeated passage of the parental Wyeth strain in mouse brains.17 The superior viral replication characteristics of Lister strain in tumor cells compared with WR in vitro and in vivo suggests greater antitumor potency against human disease and reduced virus side-effects caused by off-target replication.
High titers of WR strain virus have been reported in the ovaries of nude and immunocompetent mice following IV administration.11, 18, 19 Smallpox virus is known to replicate preferentially in areas of increased vascular permeability secondary to injury and histamine release.20 It has also been shown that increases in vascular permeability induced by hyperthermia lead to increased uptake of VV.21 Furthermore ovarian tissue has been shown to have high levels of VEGF, which increases vascular permeability and hence VV uptake.22 Research has shown partial dependence on asialo-GM1 for WR strain viruses to enter cells.23 This potential accessory receptor is widely expressed in ovarian and brain tissue, and anti-serum to asialo-GM1 has been shown to abrogate ovarian infection.23 Further studies have shown that two viral proteins, A27L and H3L, interact with heparan sulfate to infect host cells.24, 25 Heparan sulfate is found abundantly in ovarian follicular basal lamina and is a potential candidate for supporting WR virus entry into ovarian follicles.
The immune response to oncolytic viral therapy is a complex interaction, with seemingly dichotomous effects of the host immune system on tumor progression.26 One argument is that the tumor microenvironment is immunosuppressed,27 which facilitates viral replication and prolonged virally mediated oncolysis. The opposing view is that activation of the immune system in response to virus treatment leads to a bystander cytotoxic effect on cancer cells,26 as well as viral clearance. The data presented here are more supportive of the first hypothesis, in that the superior antitumor efficacy of Lister strain was associated with higher viral titers within the tumors. Furthermore, through analysis of cytokines in serum of tumor-bearing mice following vaccinia IT treatment, WR strain induced higher levels of proinflammatory cytokines IL8, IL6, IL1β, IL12, IFN-γ and lower levels of the anti-inflammatory cytokine IL-10. Therefore there is an association of heightened proinflammatory host response to WR virus with lower in vivo intratumoral viral titers and reduced antitumor efficacy compared with Lister strain virus.
The elevation of the anti-inflammatory cytokine, IL-10, in response to Lister but to a much lesser extent with WR strain treatment is of interest. IL-10 was first described as a cytokine synthesis inhibitor factor secreted by murine T-helper 2 (Th2) cells, which suppressed cytokine production by T-helper 1 (Th1) cells.28 IL-10 has broad anti-inflammatory properties through suppression of the antigen-presenting cell function and proinflammatory cytokine production of macrophages and dendritic cells.29 This anti-inflammatory action has led to investigation of the use of IL-10 in the treatment of chronic inflammatory diseases, such as Crohn’s disease, rheumatoid arthritis and psoriasis. More recently a role for IL-10 in cancer treatment has been researched with evidence of antitumor efficacy,30 and suppression of angiogenesis.31, 32, 33
IL-10 production has been demonstrated in various cell types including Th2 cells, monocytes, B-cells, eosinophils, mast cells and dendritic cells; however, the major source appears to be macrophages.34 Moreover, IL-10 has been found to be expressed by a variety of malignant tumor cell types, including breast, colorectal and lung cancers,29 and is correlated with poor prognosis. It has been shown that IL-10 contributes to tumor survival by generating a microenvironment conducive to tumor growth and metastasis.35, 36, 37, 38 The majority of tumor-associated macrophages in solid tumors express higher levels of IL-10 and lower levels of IL12 and tumor-necrosis factor α,39, 40, 41 resulting in suppression of antitumor T-cell responses.
Poxviruses are unique in their capacity to induce the release of proteins that bind cytokines, chemokines or interferons to evade the host immune response.42 There is also evidence of endogenous viral IL-10 expression by poxviruses (Yaba-like disease virus).43 Transduction of peritoneal mesothelial cells with an adenoviral vector expressing IL-10 in a gastric cancer model resulted in reduced cancer growth30 and inhibition of angiogenesis. We also have evidence that arming VVL15 with IL-10 significantly enhances the antitumor efficacy by the virus (Chard et al, unpublished data). In summary, the present study suggests that Lister strain VV with TK deletion is a potentially promising backbone for development of new cancer therapeutic agents.
Materials and methods
Cell lines
CV1, the African Green Monkey normal kidney cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). Normal human bronchial epithelial cells were obtained from Cambrex (Cambridge, UK) and maintained in bronchial epithelial growth medium (Cambrex).
The human HNSCC cell lines SCC4 (tongue) and SCC25 (tongue) were obtained from the Cancer Research UK Cell Culture Service (CRUK CCS, Clare Hall Laboratories, Potters Bar, UK) and were maintained in 1:1 DMEM and Ham’s F12 supplemented with 10% heat-inactivated FCS and 400 ng ml−1 hydrocortisone (Sigma-Aldrich, St Louis, MO, USA). The HNSCC cell lines TR126 (tongue) and TR138 (larynx) were obtained from the CRUK CCS and were maintained in DMEM and Ham’s F12 respectively, each with 10% FCS. FaDu was obtained from ATCC and maintained in Earle’s minimal essential medium with 10% FCS. The human pancreatic carcinoma cell lines SUIT2, MiaPaCa2, PANC1, PT45 and Capan1 were obtained from CRUK CCS and maintained in DMEM with 10% FCS. The human colorectal carcinoma cell lines HT29, HCT116 and SW620 were obtained from ATCC and maintained in DMEM with 10% FCS. MKN45 gastric adenocarcinoma cell line was kindly provided by Professor Stephen Mather (Barts Cancer Institute, Queen Mary University of London, UK) and maintained in DMEM with 10% FCS. The human ovarian carcinoma cell line A2780 was kindly provided by Professor Iain McNeish (Institute of Cancer Sciences, University of Glasgow) and maintained in DMEM with 10% FCS. All human cell lines were genotyped by STR and confirmed their identification.
The murine HNSCC cell line SCCVII was kindly provided by Dr Osam Mazda (Department of Microbiology, Kyoto Prefectural University of Medicine, Japan) and maintained in DMEM supplemented with 10% FCS. The murine pancreatic carcinoma cell line PANC02 was obtained from the ATCC and maintained in DMEM with 10% FCS. The murine colorectal carcinoma cell lines CT26 and CMT93 was obtained from CRUK CSS and maintained in DMEM supplemented with 10% FCS. MOSEC ovarian carcinoma cell line was kindly provided by Professor Iain McNeish (Barts Cancer Institute, Queen Mary University of London, UK) and maintained in DMEM with 1% Insulin-Transferrin-Selenium supplement-G (GIBCO, Invitrogen) and 3% FCS.
Viruses
VVL15 was constructed by the insertion of the lacZ reporter and the firefly luciferase genes into the TK region of the Lister vaccine strain of vaccinia virus (VVLister) under the control of the synthetic early/late and p7.5 promoters respectively,18 using an in vitro intracellular recombination technique previously described.44 The virus was kindly provided by Professor Istvan Fodor (Loma Linda University, Loma Linda, CA, USA).
WR, TK deletion vaccinia virus and WRDD, double deletion (TK and VGF) vaccinia virus were kindly provided by Dr Steve Thorne (University of Pittsburgh, Pittsburgh, PA, USA) and Dr Andrea McCart (University of Toronto, Toronto, ON, Canada) respectively.11, 19
Evaluation of viral cytotoxicity in vitro
Cells were seeded at 1 × 103 and 1 × 104 cells per well, depending on growth rates, in 96-well plates, and infected with viruses 16–18 h later. Cell survival on day 6 after viral infection was determined by MTS assay and EC50 value (viral dose killing 50% of tumor cells) was calculated as previously described.45 All assays were performed at least three times.
Viral replication
Cells were seeded at 2–4 × 105 cells per well, depending on growth rates, in three wells of six-well plates in media with 10% FCS, and infected with 1 PFU cell−1 of vaccinia viruses 16–18 h later. Samples were harvested in triplicate at 24-h intervals up to 144 h. Viral replication was detected by TCID50 (50% tissue culture infective dose) as previously described.45
ELISA
Tumor and serum samples were generated and processed as described in other sections of the methods. The concentration of cytokines was determined by using a Meso Scale Discovery multispot assay system mouse proinflammatory 7-plex ultra-sensitive kit (MSD Gaithersburg, Maryland, USA) following the manufacturer’s instructions, and 1:2 dilutions were used, with samples tested in triplicate.
In vivo efficacy experiments
Flank tumors were established in 10 mice per treatment group through subcutaneous injection of 1–5 × 106 murine and human cancer cells and allowed to reach 0.4–0.5 cm in diameter, then the mice were regrouped by tumor size and received three 50 μl IT injections of 1 × 107 PFU (nude mice) or 1 × 108 PFU (immunocompetent mice) or PBS on days 1, 3 and 5 or days 1, 2, 3, 4 and 5. Tumor volumes were calculated (volume=(length × width2 × π)/6) twice weekly until mice were sacrificed when tumor volume reached 1.00 cm3 or had been present for 3 months. 4–5-week-old female nude mice strains BALB/c and ICRF, and immunocompetent mice strains BALB/c and C57BL/6 were obtained from Harlan UK Ltd (Bicester, Oxon, UK).
Biological time point studies
Nude mouse models
5 × 106 CMT93 cells were implanted subcutaneously into the right flank of 36 ICRF nude female 4–5-week-old mice (Harlan UK Ltd). When tumors reached 0.4–0.5 cm in diameter, mice were regrouped into 12 mice per group to ensure even spread of tumor sizes. Each group received 50-μl IT injections of 1 × 107 PFU VVL15, WRDD or PBS on days 1, 3 and 5. On days 5, 10, 15 and 20 three mice were sacrificed from each group, and the tumors were harvested and snap frozen in liquid nitrogen for further processing. Blood samples were collected in heparinised tubes prior to freezing.
The experiment was repeated with PT45 cells using the same method, except mice were sacrificed on days 7, 12 and 20.
Immunocompetent mouse models
The experiment was further repeated in C57BL/6 immunocompetent mice implanted with CMT93 cells. 50-μl IT injections of 1 × 108 PFU and PBS were performed on days 1, 3 and 5. Mice were sacrificed on days 5, 10, 15 and 20.
Biodistribution experiments in the nude mouse model
5 × 106 CMT93 cells were implanted subcutaneously into the right flank of 60 ICRF nude female 4–5-week-old mice (Harlan UK Ltd). When tumors reached 0.4–0.5 cm in diameter, mice were regrouped into 12 mice per group to ensure even spread of tumor sizes. Each group received a single 100-μl IV tail vein injections of 1 × 107 PFU VVL15, WRDD or PBS. On days 2, 6, 8 and 12 three mice were sacrificed from each group and the tumors and other tissues (ovary, bone marrow, liver, lungs, brain and spleen) were harvested and snap frozen in liquid nitrogen for further processing.
Biodistribution experiments in the immunocompetent mouse model
5 × 106 CMT93 cells were implanted subcutaneously into the right flank of 36 C57BL/6 female 4–5-week-old mice (Harlan UK Ltd). When tumors reached 0.4–0.5 cm in diameter, mice were regrouped into 9 mice per group to ensure even spread of tumor sizes. Each group received 100 μl IV tail vein injections of 2 × 108 PFU VVL15, WRDD or PBS. On days 4, 8 and 12 three mice were sacrificed from each group and the tumors and other tissues (ovary, bone marrow, liver, lungs, brain, blood and spleen) were harvested and snap frozen in liquid nitrogen for further processing. Blood samples were collected in heparinized tubes prior to freezing.
In vivo virus quantification
Tissue processing
Mouse tissue was weighed, homogenized to a total volume of 2 ml in DMEM, then frozen and thawed three times in liquid nitrogen and in a water bath at 37 °C respectively.
TCID50
Vaccinia virus titration in homogenized mouse tumor and organ samples was performed using a TCID50 technique as previously described. As with the in vitro samples, 20 μl of homogenized tissue was added to the first row of seeded CV1 cells and following a change of pipette tips, serial 1:10 dilutions were made to the next seven consecutive rows. Viral titers were corrected for the initial weight of the sample.
Quantitative Real-Time PCR (qRT-PCR)
100 μl of homogenized tissue was used for DNA extraction using the DNeasy 96 blood and tissue kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions, and eluted in 100 μl of buffer AE. DNA concentration was determined by using the NanoDrop ND-1000 Spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA) and a fixed amount of DNA was used for qRT-PCR. DNA was accepted as adequately pure where the ratio of absorbance at 260 and 280 nm was ~1.8. DNA copies were against total DNA and the results are displayed as arbitrary units. The results are displayed as PFU ml−1 g−1, normalized against the weight of the tissue sample. Primers and probes for VVLister were designed manually using Primer Express v3.0 software (Applied Biosystems, Hammonton, NJ, USA) and constructed by Sigma-Aldrich and Applied Biosystems respectively.
Samples, control (RNAse free water) and nine 10-fold serial dilutions of standards (5 × 108 to five viral genome copies diluted in 5-μl RNAse free water) were tested in triplicate in each plate by qRT-PCR. A 25-μl reaction volume consisted of 5-μl sample or standard and 20 μl of Master mix (0.9-μm forward primer, 0.9-μm reverse primer, 0.2-μm probe in TaqMan Universal PCR Master Mix, Applied Biosystems). Reactions were performed in MicroAmp optical 96-well reaction plates sealed with optical adhesive covers and amplified by using the 7500 Real-time PCR System (1 cycle of 48 °C for 30 min, 95 °C for 10 min then 40 cycles of 95 °C for 15 s, 60 °C for 1 min). Cycle thresholds (CT) were determined using 7500 System SBS software (all Applied Biosystems).
Immunohistochemistry staining
All tissues were processed for histopathology and IHC analysis for viral coat protein (1:2000 rabbit anti-vaccinia virus coat protein polyclonal antibody (MorphoSys UK Ltd, Bath, UK).
Statistical analysis
All statistical analyses were performed as described in Figure legends or results. Differences were considered significant where P<0.05.
References
Wong HH, Lemoine NR, Wang Y . Oncolytic viruses for cancer therapy: overcoming the obstacles. Viruses 2010; 2: 78–106.
Garber K . China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst 2006; 98: 298–300.
Mathis JM, Stoff-Khalili MA, Curiel DT . Oncolytic adenoviruses – selective retargeting to tumor cells. Oncogene 2005; 24: 7775–7791.
Kirn DH, Thorne SH . Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer 2009; 9: 64–71.
Friedman GK, Haas MC, Kelly VM, Markert JM, Gillespie GY, Cassady KA . Hypoxia moderates gamma(1)34.5-deleted herpes simplex virus oncolytic activity in human glioma xenoline primary cultures. Transl Oncol 2012; 5: 200–207.
Shen B, Bauzon M, Hermiston T . The effect of hypoxia on the uptake, replication and lytic potential of group B adenovirus type 3 (Ad3) and type 11p (Ad11p). Gene Therapy 2006; 13: 986–990.
Shen B, Hermiston T . Effect of hypoxia on Ad5 infection, transgene expression and replication. Gene Therapy 2005; 12: 902–910.
Hiley CT, Yuan M, Lemoine NR, Wang Y . Lister strain vaccinia virus, a potential therapeutic vector targeting hypoxic tumours. Gene Therapy 2010; 17: 281–287.
Fenner F, HD AI, Jezek Z, Ladnyi ID . Smallpox and its Eradication. WHO: Geneva, 1988.
Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest 2007; 117: 3350–3358.
McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res 2001; 61: 8751–8757.
Tysome JR, Briat A, Alusi G, Cao F, Gao D, Yu J et al. Lister strain of vaccinia virus armed with endostatin-angiostatin fusion gene as a novel therapeutic agent for human pancreatic cancer. Gene Ther 2009; 16: 1223–1233.
Tysome JR, Wang P, Alusi G, Briat A, Gangeswaran R, Wang J et al. Lister vaccine strain of vaccinia virus armed with the endostatin-angiostatin fusion gene: an oncolytic virus superior to dl1520 (ONYX-015) for human head and neck cancer. Hum Gene Ther 2011; 22: 1101–1108.
Haddad D, Chen N, Zhang Q, Chen CH, Yu YA, Gonzalez L et al. A novel genetically modified oncolytic vaccinia virus in experimental models is effective against a wide range of human cancers. Ann Surg Oncol 2012; 19: S665–S674.
Bahar MW, Graham SC, Chen RA, Cooray S, Smith GL, Stuart DI et al. How vaccinia virus has evolved to subvert the host immune response. J Struct Biol 2011; 175: 127–134.
Smith GL, Symons JA, Khanna A, Vanderplasschen A, Alcami A . Vaccinia virus immune evasion. Immunol Rev 1997; 159: 137–154.
Williamson JD, Reith RW, Jeffrey LJ, Arrand JR, Mackett M . Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J Gen Virol 1990; 71: 2761–2767.
Hung CF, Tsai YC, He L, Coukos G, Fodor I, Qin L et al. Vaccinia virus preferentially infects and controls human and murine ovarian tumors in mice. Gene Ther 2007; 14: 20–29.
Puhlmann M, Brown CK, Gnant M, Huang J, Libutti SK, Alexander HR et al. Vaccinia as a vector for tumor-directed gene therapy: biodistribution of a thymidine kinase-deleted mutant. Cancer Gene Ther 2000; 7: 66–73.
Ricketts TF . The diagnosis of smallpox. U. S. Public Health Service, Division of Foreign Quarantine: Washington, 1966.
Chang E, Chalikonda S, Friedl J, Xu H, Phan GQ, Marincola FM et al. Targeting vaccinia to solid tumors with local hyperthermia. Hum Gene Ther 2005; 16: 435–444.
Reynolds LP, Grazul-Bilska AT, Redmer DA . Angiogenesis in the corpus luteum. Endocrine 2000; 12: 1–9.
Karupiah G, Blanden RV . Anti-asialo-GM1 inhibits vaccinia virus infection of murine ovaries: asialo-GM1 as an additional virus receptor? Immunol Cell Biol 1990; 68: 343–346.
Chung CS, Hsiao JC, Chang YS, Chang W . A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J Virol 1998; 72: 1577–1585.
Lin CL, Chung CS, Heine HG, Chang W . Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J Virol 2000; 74: 3353–3365.
Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther 2009; 20: 1119–1132.
Zitvogel L, Tesniere A, Kroemer G . Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 2006; 6: 715–727.
Fiorentino DF, Bond MW, Mosmann TR . Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med 1989; 170: 2081–2095.
O'Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C . Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev 2008; 223: 114–131.
Tanaka F, Tominaga K, Shiota M, Ochi M, Kuwamura H, Tanigawa T et al. Interleukin-10 gene transfer to peritoneal mesothelial cells suppresses peritoneal dissemination of gastric cancer cells due to a persistently high concentration in the peritoneal cavity. Cancer Gene Ther 2008; 15: 51–59.
Kohno T, Mizukami H, Suzuki M, Saga Y, Takei Y, Shimpo M et al. Interleukin-10-mediated inhibition of angiogenesis and tumor growth in mice bearing VEGF-producing ovarian cancer. Cancer Res 2003; 63: 5091–5094.
Cervenak L, Morbidelli L, Donati D, Donnini S, Kambayashi T, Wilson JL et al. Abolished angiogenicity and tumorigenicity of Burkitt lymphoma by interleukin-10. Blood 2000; 96: 2568–2573.
Huang S, Ullrich SE, Bar-Eli M . Regulation of tumor growth and metastasis by interleukin-10: the melanoma experience. J Interferon Cytokine Res 1999; 19: 697–703.
Spits H . de Waal Malefyt R. Functional characterization of human IL-10. Int Arch Allergy Immunol 1992; 99: 8–15.
Yue FY, Dummer R, Geertsen R, Hofbauer G, Laine E, Manolio S et al. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. Int J Cancer 1997; 71: 630–637.
Matsuda M, Salazar F, Petersson M, Masucci G, Hansson J, Pisa P et al. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med 1994; 180: 2371–2376.
Masood R, Zhang Y, Bond MW, Scadden DT, Moudgil T, Law RE et al. Interleukin-10 is an autocrine growth factor for acquired immunodeficiency syndrome-related B-cell lymphoma. Blood 1995; 85: 3423–3430.
Gu ZJ, Costes V, Lu ZY, Zhang XG, Pitard V, Moreau JF et al. Interleukin-10 is a growth factor for human myeloma cells by induction of an oncostatin M autocrine loop. Blood 1996; 88: 3972–3986.
Sica A, Bronte V . Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 2007; 117: 1155–1166.
Sica A, Schioppa T, Mantovani A, Allavena P . Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer 2006; 42: 717–727.
Porta C, Subhra Kumar B, Larghi P, Rubino L, Mancino A, Sica A . Tumor promotion by tumor-associated macrophages. Adv Exp Med Biol 2007; 604: 67–86.
Smith GL . Vaccinia virus immune evasion. Immunol Lett 1999; 65: 55–62.
Bartlett NW, Dumoutier L, Renauld JC, Kotenko SV, McVey CE, Lee HJ et al. A new member of the interleukin 10-related cytokine family encoded by a poxvirus. J Gen Virol 2004; 85: 1401–1412.
Timiryasova TM, Chen B, Fodor N, Fodor I . Construction of recombinant vaccinia viruses using PUV-inactivated virus as a helper. Biotechniques 2001; 31: 538–540.
Wang Y, Hallden G, Hill R, Anand A, Liu TC, Francis J et al. E3 gene manipulations affect oncolytic adenovirus activity in immunocompetent tumor models. Nat Biotechnol 2003; 21: 1328–1335.
Acknowledgements
The provision of WRLuc virus by Steve Thorne is greatly appreciated. We acknowledge the assistance of Jennelle Francis in virus production, and Gary Martin in helping with the animal experiments. All histology and immunohistochemistry was performed by the Barts Cancer Institute Pathology Service. This project was supported by Barts and The London Charity, Ministry of Science and Technology, China (2013DFG32080), Henan Provincial Department of Science and Technology as well as Department of Health, Henan Province, China (124200510018 and 104300510008) and The Royal College of Surgeons of England.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Hughes, J., Wang, P., Alusi, G. et al. Lister strain vaccinia virus with thymidine kinase gene deletion is a tractable platform for development of a new generation of oncolytic virus. Gene Ther 22, 476–484 (2015). https://doi.org/10.1038/gt.2015.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.13
This article is cited by
-
Building sophisticated sensors of extracellular cues that enable mammalian cells to work as “doctors” in the body
Cellular and Molecular Life Sciences (2020)
-
A marker-free system for highly efficient construction of vaccinia virus vectors using CRISPR Cas9
Molecular Therapy - Methods & Clinical Development (2015)