Abstract
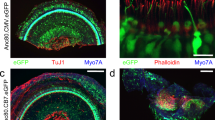
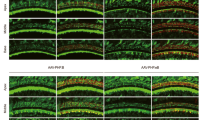
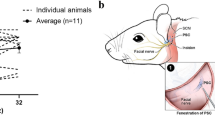
The auditory portion of the inner ear, the cochlea, is an ideal organ for local gene transfection owing to its relative isolation. Various carriers have been tested for cochlear gene transfection. To date, viral vectors appear to have much higher transfection efficacy than non-viral mechanisms. Among these vectors, recombinant adeno-associated virus (rAAV) vectors have several advantages such as being non-pathogenic and the ability to produce prolonged gene expression in various cell types. However, rAAV vectors cannot pass through the intact round window membrane (RWM), otherwise a very attractive approach to access the human inner ear. In this study, performed in guinea-pigs, we describe a method to increase the permeability of RWM to rAAV vectors by partial digestion with collagenase solution. Elevated delivery of rAAV across the partially digested RWM increased transfection efficacy to a satisfactory level, even though it was still lower than that achieved by direct cochleostomy injection. Functional tests (auditory brainstem responses) showed that this enzymatic manipulation did not cause permanent hearing loss if applied appropriately. Morphological observations suggested that the damage to RWM caused by partial digestion healed within four weeks. Taken together, these findings suggest that partial digestion of the RWM is a safe and effective method for increasing the transfection of cochlear sensory cells with rAAV.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Duan M, Venail F, Spencer N, Mezzina M . Treatment of peripheral sensorineural hearing loss: gene therapy. Gene Therapy 2004; 11 (Suppl 1): S51–S56.
Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med 2005; 11: 271–276.
Hildebrand MS, Newton SS, Gubbels SP, Sheffield AM, Kochhar A, de Silva MG et al. Advances in molecular and cellular therapies for hearing loss. Mol Ther 2008; 16: 224–236.
Brigande JV, Heller S . Quo vadis, hair cell regeneration? Nat Neurosci 2009; 12: 679–685.
Kesser BW, Lalwani AK . Gene therapy and stem cell transplantation: strategies for hearing restoration. Adv Otorhinolaryngol 2009; 66: 64–86.
Maeda Y, Sheffield AM, Smith RJ . Therapeutic regulation of gene expression in the inner ear using RNA interference. Adv Otorhinolaryngol 2009; 66: 13–36.
Wei D, Yamoah EN . Regeneration of the mammalian inner ear sensory epithelium. Curr Opin Otolaryngol Head Neck Surg 2009; 17: 373–380.
Cotanche DA . Genetic and pharmacological intervention for treatment/prevention of hearing loss. J Commun Disord 2008; 41: 421–443.
Luebke AE, Rova C, Von Doersten PG, Poulsen DJ . Adenoviral and AAV-mediated gene transfer to the inner ear: role of serotype, promoter, and viral load on in vivo and in vitro infection efficiencies. Adv Otorhinolaryngol 2009; 66: 87–98.
Jero J, Mhatre AN, Tseng CJ, Stern RE, Coling DE, Goldstein JA et al. Cochlear gene delivery through an intact round window membrane in mouse. Hum Gene Ther 2001; 12: 539–548.
Suzuki M, Yagi M, Brown JN, Miller AL, Miller JM, Raphael Y . Effect of transgenic GDNF expression on gentamicin-induced cochlear and vestibular toxicity. Gene Therapy 2000; 7: 1046–1054.
Konishi T, Salt AN, Hamrick PE . Effects of exposure to noise on permeability to potassium of the endolymph–perilymph barrier in guinea pigs. Acta Otolaryngol 1982; 94: 395–401.
Stover T, Yagi M, Raphael Y . Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res 1999; 136: 124–130.
Carvalho GJ, Lalwani AK . The effect of cochleostomy and intracochlear infusion on auditory brain stem response threshold in the guinea pig. Am J Otol 1999; 20: 87–90.
Salt AN, Sirjani DB, Hartsock JJ, Gill RM, Plontke SK . Marker retention in the cochlea following injections through the round window membrane. Hear Res 2007; 232: 78–86.
Kaplan DM, Hehar SS, Bance ML, Rutka JA . Intentional ablation of vestibular function using commercially available topical gentamicin–betamethasone eardrops in patients with Meniere's disease: further evidence for topical eardrop ototoxicity. Laryngoscope 2002; 112: 689–695.
Bath AP, Walsh RM, Bance ML . Presumed reduction of vestibular function in unilateral Meniere's disease with aminoglycoside eardrops. J Laryngol Otol 1999; 113: 916–918.
Bath AP, Walsh RM, Bance ML, Rutka JA . Ototoxicity of topical gentamicin preparations. Laryngoscope 1999; 109: 1088–1093.
Wang Y, Hirose K, Liberman MC . Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol 2002; 3: 248–268.
Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA 2008; 105: 7827–7832.
Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther 2009; 17: 463–471.
Silverstein H, Arruda J, Rosenberg SI, Deems D, Hester TO . Direct round window membrane application of gentamicin in the treatment of Meniere's disease. Otolaryngol Head Neck Surg 1999; 120: 649–655.
Luebke AE, Foster PK, Muller CD, Peel AL . Cochlear function and transgene expression in the guinea pig cochlea, using adenovirus- and adeno-associated virus-directed gene transfer. Hum Gene Ther 2001; 12: 773–781.
Konishi M, Kawamoto K, Izumikawa M, Kuriyama H, Yamashita T . Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J Gene Med 2008; 10: 610–618.
Liu YH, Ke XM, Qin Y, Gu ZP, Xiao SF . Adeno-associated virus-mediated Bcl-xL prevents aminoglycoside-induced hearing loss in mice. Chin Med J (Engl) 2007; 120: 1236–1240.
Cooper LB, Chan DK, Roediger FC, Shaffer BR, Fraser JF, Musatov S et al. AAV-mediated delivery of the caspase inhibitor XIAP protects against cisplatin ototoxicity. Otol Neurotol 2006; 27: 484–490.
Romano G . Current development of adeno-associated viral vectors. Drug News Perspect 2005; 18: 311–316.
Stone IM, Lurie DI, Kelley MW, Poulsen DJ . Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther 2005; 11: 843–848.
Carson SD . Limited proteolysis of the coxsackievirus and adenovirus receptor (CAR) on HeLa cells exposed to trypsin. FEBS Lett 2000; 484: 149–152.
Soudais C, Boutin S, Hong SS, Chillon M, Danos O, Bergelson JM et al. Canine adenovirus type 2 attachment and internalization: coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathway. J Virol 2000; 74: 10639–10649.
Qiu J, Handa A, Kirby M, Brown KE . The interaction of heparin sulfate and adeno-associated virus 2. Virology 2000; 269: 137–147.
Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA . Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol 2001; 75: 6884–6893.
Samulski RJ, Zhu X, Xiao X, Brook JD, Housman DE, Epstein N et al. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J 1991; 10: 3941–3950.
Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, Laughlin CA et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA 1990; 87: 2211–2215.
Goycoolea MV, Lundman L . Round window membrane. Structure function and permeability: a review. Microsc Res Technol 1997; 36: 201–211.
Shi L, Ermis R, Garcia A, Telgenhoff D, Aust D . Degradation of human collagen isoforms by Clostridium collagenase and the effects of degradation products on cell migration. Int Wound J 2010; 7: 87–95.
Yoshihara T, Kaname H, Ishii T, Igarashi M . Subepithelial fiber components of the round window membrane of the guinea pig: an ultrastructural and immunohistochemical study. ORL J Otorhinolaryngol Relat Spec 1995; 57: 115–121.
Lalwani A, Walsh B, Reilly P, Carvalho G, Zolotukhin S, Muzyczka N et al. Long-term in vivo cochlear transgene expression mediated by recombinant adeno-associated virus. Gene Therapy 1998; 5: 277–281.
Liu Y, Okada T, Sheykholeslami K, Shimazaki K, Nomoto T, Muramatsu S et al. Specific and efficient transduction of cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol Ther 2005; 12: 725–733.
Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 2004; 10: 302–317.
Fritzsch B, Farinas I, Reichardt LF . Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci 1997; 17: 6213–6225.
Liu Y, Okada T, Nomoto T, Ke X, Kume A, Ozawa K et al. Promoter effects of adeno-associated viral vector for transgene expression in the cochlea in vivo. Exp Mol Med 2007; 39: 170–175.
Boeda B, Weil D, Petit C . A specific promoter of the sensory cells of the inner ear defined by transgenesis. Hum Mol Genet 2001; 10: 1581–1589.
Summerford C, Samulski RJ . Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol 1998; 72: 1438–1445.
Acknowledgements
This study was supported by grants from Natural Outstanding Youth Foundation of China (Grant No. 30925035), Special Program for Key Basic Research of the Ministry of Science and Technology, China (Grant No. 2009CB526504) and National Natural Science Foundation of China (Grant No. 30901669).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, H., Murphy, R., Taaffe, D. et al. Efficient cochlear gene transfection in guinea-pigs with adeno-associated viral vectors by partial digestion of round window membrane. Gene Ther 19, 255–263 (2012). https://doi.org/10.1038/gt.2011.91
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2011.91
Keywords
This article is cited by
-
Gene therapy development in hearing research in China
Gene Therapy (2020)
-
Intravenous rAAV2/9 injection for murine cochlear gene delivery
Scientific Reports (2017)
-
Cochlear gene therapy with ancestral AAV in adult mice: complete transduction of inner hair cells without cochlear dysfunction
Scientific Reports (2017)
-
In vivo genetic manipulation of inner ear connexin expression by bovine adeno-associated viral vectors
Scientific Reports (2017)
-
Hyaluronic acid pretreatment for Sendai virus-mediated cochlear gene transfer
Gene Therapy (2016)