Abstract
Purpose
To evaluate the effect of orbital reconstruction and factors related to the effect of orbital reconstruction by assessing of orbital volume using orbital computed tomography (CT) in cases of orbital wall fracture.
Methods
In this retrospective study, 68 patients with isolated blowout fractures were evaluated. The volumes of orbits and herniated orbital tissues were determined by CT scans using a three-dimensional reconstruction technique (the Eclipse Treatment Planning System). Orbital CT was performed preoperatively, immediately after surgery, and at final follow ups (minimum of 6 months). We evaluated the reconstructive effect of surgery making a new formula, ‘orbital volume reconstruction rate’ from orbital volume differences between fractured and contralateral orbits before surgery, immediately after surgery, and at final follow up.
Results
Mean volume of fractured orbits before surgery was 23.01±2.60 cm3 and that of contralateral orbits was 21.31±2.50 cm3 (P=0.005). Mean volume of the fractured orbits immediately after surgery was 21.29±2.42 cm3, and that of the contralateral orbits was 21.33±2.52 cm3 (P=0.921). Mean volume of fractured orbits at final follow up was 21.50±2.44 cm3, and that of contralateral orbits was 21.32±2.50 cm3 (P=0.668). The mean orbital volume reconstruction rate was 100.47% immediately after surgery and 99.17% at final follow up. No significant difference in orbital volume reconstruction rate was observed with respect to fracture site or orbital implant type. Patients that underwent operation within 14 days of trauma had a better reconstruction rate at final follow up than patients who underwent operation over 14 days after trauma (P=0.039).
Conclusion
Computer-based measurements of orbital fracture volume can be used to evaluate the reconstructive effect of orbital implants and provide useful quantitative information. Significant reduction of orbital volume is observed immediately after orbital wall reconstruction surgery and the reconstruction effect is maintained for more than minimum 6 months. Patients that undergo surgery within 14 days of trauma has better reconstruction rates at final follow up, which supports the need for early surgery.
Similar content being viewed by others
Introduction
The orbital floor and medial wall are susceptible to trauma and are common fracture sites. When an orbital fracture causes complications, such as, enophthalmos, impaired ocular motility or diplopia, orbital wall reconstruction is needed.1 The effect of orbital wall reconstruction is frequently estimated based on enophthalmos improvement. Various causes of enophthalmos associated with orbital wall fracture have been suggested, such as increased orbital volume due to displacement of bony orbit and orbital tissue, necrosis and fibrosis of orbital soft tissue, and dislocation of an intra-orbital supporting tendon.2 Among these, increased orbital volume is considered the main cause and thus a number of recent studies have measured orbital volumes and used them to assess the efficacy of orbital wall reconstruction.2, 3, 4, 5, 6, 7 However, these studies were limited by small subject numbers (under 30 patients) and/or short follow-up periods (under 6 months). Furthermore, various aspects, such as, type of orbital implant, orbital wall fracture location, time elapsed between trauma and surgical intervention, and quantification of the effect of orbital wall reconstruction were not considered. In addition, in the majority of studies orbital volume measurements were performed before and after surgery only, and thus the maintenance effect of orbital wall reconstruction was not estimated with the time course.
To address these limitations, we measured orbital volumes by computed tomography (CT) using a three-dimensional imaging software program (The Eclipse Treatment Planning System ver.13.0; Varian Medical Systems, Inc., Palo Alto, CA, USA) at three time points, that is, before surgery, immediately after surgery, and at final follow up (at least 6 months after surgery), using measured fractured and contralateral orbit volumes. In addition, we sought to evaluate the effect of orbital reconstruction quantitatively and to identify factors related to the effect of orbital reconstruction on orbital volume.
Materials and methods
From April 2010 to March 2015, a total of 68 patients with a unilateral orbital blowout fracture treated surgically at Department of Ophthalmology, Gachon University Gil Medical Center and followed for at least 6 months were identified and included in this study. Gachon University IRB committee approved this study. This research adheres to the tenets of the Declaration of Helsinki. The exclusion criteria applied were; bilateral orbital wall fractures, orbital wall fractures combined with another facial bone fracture, thyroid associated ophthalmopathy, and orbital tumor, all of which can cause orbital volume measurement errors. Before surgery, the following tests were performed; visual acuity, a slit lamp examination, pupillary reflex test, fundus examination by indirect ophthalmoscopy, the Goldmann diplopia test by perimetry, extraocular motility test, and Hertel exophthalmometry to measure enophthalmos; and infraorbital nerve hypoesthesia was identified. In addition, the location and extent of orbital wall fractures and entrapment of extraocular muscle or soft tissue were identified by CT.
Surgery was performed by one surgeon under general anesthesia. Fractures were exposed through a transconjunctival incision for inferior orbital wall fractures, and through a transcaruncular incision for medial orbital wall fractures. Herniated orbital contents were repositioned by a periosteal elevator, a suction tip, and a malleable retractor. Orbital wall defects were reconstructed using orbital implants (cut to fit the anatomies of fracture sites and soaked in saline mixed with antibiotics) by insertion under periosteum at fracture sites. After this procedure, a forced duction test was performed to ensure the absence of restriction. During surgery pupil size was checked for optic nerve compression. To reduce orbital tissue swelling, methylprednisolone (250 mg) was administered intravenously during surgery, after surgery, and the day after surgery, respectively. Thereafter, methylprednisolone was given orally and tapered. During the first month after surgery, patients were observed weekly and then monthly. For all 68 patients, the observation period was more than six months.
Degrees of clinical improvement were assessed by grading clinical signs at 1, 3, and 6 months after surgery, as follows, diplopia within the central 20° visual field as grade III, diplopia between 20° and 40° visual fields as grade II, diplopia only on the periphery beyond the 40° visual field as grade I, and ‘no diplopia’ was defined as the absence of diplopia. Extraocular movement limitation was assessed using the position of corneal reflection using pen light illumination of the cornea with the eye looking in the direction of extraocular muscle action. Severe limitation with inability to move the eye was defined as a limitation of −4; moderate limitation with corneal reflection in contrary pupil margin as a limitation of −3; moderate limitation with corneal reflection between the contrary pupil margin and limbus as a limitation of −2; mild limitation with corneal reflection in contrary limbus as a limitation of −1; and no limitation with a pen light reflection position beyond the limbus as a limitation of 0.
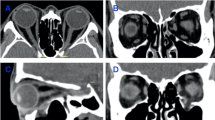
CT examinations were performed on all study subjects before surgery, immediately after surgery, and at final follow up. Locations and ranges of orbital wall fractures and strangulation of orbital soft tissues or extraocular muscles were identified before surgery by CT. Locations of orbital implants and reductions of fractured orbits and herniated orbital tissues were identified immediately after surgery, and orbital wall reconstruction maintenance was assessed at final follow up. A CT scanning system, ‘The Eclipse Treatment Planning System’ (ver.13.0, Varian Medical System, Inc.) was used to measure volumes of fractured and contralateral orbits before and after surgery. In each case, bony orbital and herniated orbital tissue areas on sections were measured using a drawing cursor freehand. Drawn orbital contours were then reconstructed as three-dimensional images and orbital volumes were calculated automatically. This program is straightforward to use and provides low errors and high intra- and inter-operator reproducibilities. The anterior orbital boundary was defined by a straight line connecting the medial and lateral orbital rims, and the posterior orbital boundary was defined as the orbital apex on axial CT scans. To reduce measurement errors, orbital volumes were measured three times by one investigator and then averaged (Figure 1). The effect of orbital wall reconstruction was quantified using orbital volume reconstruction rate (OVR%), which was defined as follows:
Example of orbital volume measurement using ‘The Eclipse Treatment Planning System (Ver 13.0, Varian)’. Axial plane: the anterior orbital boundary was defined by a straight line connecting the medial and the lateral orbital rims, and the posterior limit was defined as the orbital apex. Coronal plane: the anterior orbital boundary was defined as the CT slice in which 50% of the inferior orbital rim was visible, and the posterior limit was defined as the orbital apex. Sagittal plane: the anterior orbital boundary was defined by the straight line connecting the superior and inferior orbital rims, and the posterior limit was defined as the orbital apex. The areas of these outlines were measured on each scan and summed to obtain orbital volumes. (a) Preoperative. The red arrow indicates a left inferior wall fracture and herniated orbital tissue before surgery. (b) Postoperative. The green arrow indicates reconstructed left inferior wall fracture. Postoperative image shows reduction of the displaced orbital wall and herniated orbital tissue.

A: volume of fractured orbit after surgery. B: volume of contralateral orbit.
That is, when fractured orbit volume after surgery equals that of the contralateral orbit, OVR% is 100%, and when fractured orbit volume after surgery is 10% larger than that of the contralateral orbit then OVR%=90%.
Data were statistically described in terms of range, mean±SD, and percentages when appropriate. Comparison of numeric variables between the study groups was done using Student’s t-test or ANOVA (Analysis Of Variance) for independent samples, whereas within group comparison of numeric variables was performed using the paired Student’s t-test. All P-values <0.05 was considered statistically significant. All statistical calculations were done using SPSS (Statistical Package for the Social Science; SPSS, Inc., Chicago, IL, USA) version 18 for the Microsoft Windows.
Results
The study included 68 patients and mean follow up was 15.2±4.03 months. Forty eight patients were male (70.6%) and 20 were female (29.4%). Ages ranged from 7 to 68 years and mean patient age was 35.4 years. Nine patients (13.2%) were aged under 20 years, 17 patients (25.0%) were between 21 and 30, 19 (27.9%) were between 31 and 40, 15 (22.1%) were between 41 and 50, and 8 (11.8%) patients were over 50 years old. Patients were categorized into 3 groups based on anatomic fracture location as determined by CT, as follows; 30 patients (44.1%) to an inferior wall fracture group, 21 patients (30.9%) to a medial wall fracture group, and 17 (25.0%) to an inferior and medial wall fracture group. Mean time from trauma to surgical repair was 7.03 days (range 1–17 days). Limitation of extraocular muscle movement test result was observed in 13 patients before surgery. These extraocular muscle movement limitations were improved in all cases at final follow up. Fourteen patients appealed diplopia before surgery, five patients for grade I, four patients for grade II, and five patients for grade 3, and this was improved in all at final follow up. Mean enophthalmos for all study subjects was 0.4±1.0 mm before surgery and 0.1±0.3 mm after surgery, and mean enophthalmos for 7 patients with definite (>2 mm) enophthalmos or who were expected to have definite enophthalmos in the future time was 2.5±0.8 mm before surgery and 0.4±0.2 mm after surgery. These results confirmed enophthalmos improvement. Eight patients described infraorbital hypoesthesia before surgery, and this was improved in all at final follow up. No orbital implant related infection, dislocation or exposure, or visual loss caused by orbital implant related problems was observed during follow up. Mean volume of fractured orbits before surgery was 23.01±2.60 cm3 and that of contralateral orbits was 21.31±2.50 cm3 (P=0.005). Immediately after surgery, mean volume of fractured orbits was 21.29±2.42 cm3 and that of contralateral orbits was 21.33±2.52 cm3 (P=0.921), and mean volume of fractured orbits at final follow up was 21.50±2.44 cm3 and that of contralateral orbits was 21.32±2.50 cm3 (P=0.668, Table 1). Mean volume of fractured orbit was significantly smaller immediately after surgery than that measured before surgery (P=0.004). There was no significant difference between the mean volumes of fractured orbit immediately after surgery and at final follow up (P=0.561). OVR% of orbital wall fractures was 100.47% immediately after surgery and 99.17% at final follow up (P=0.891).
No significant intergroup difference of OVR% was observed among groups defined by fractured site or by type of orbital implant immediately after surgery and at last follow up (Tables 2 and 3). Patients that underwent surgery at ≤14 or >14 days after injury showed no significant OVR% difference immediately after surgery (P=0.891, Table 4), but those that underwent surgery at ≤14 days after surgery had a better mean OVR% at final follow up (P=0.039, Table 4).
Discussion
The goal of the surgery for orbital wall fracture is the reduction of herniated orbital tissues and of the fractured bony orbit and to correct diplopia, enophthalmos, and limitations of extraocular muscle movement.1 Although some controversy exists about the surgical indications of orbital wall fracture, traditional indications are the presence of symptoms and signs, such as, diplopia of duration >2 weeks, limitation of extraocular muscle movement, radiologic evidence of extensive fracture, and enophthalmos produced by an orbital volume change.8, 9
There is no difficulty determining whether surgery is necessary if surgical indications, such as, severe diplopia or extraocular muscle movement limitation, or prominent enophthalmos are definite in cases of orbital blowout fracture, but when such symptoms and signs are insignificant, it is difficult to determine whether or when surgery is indicated. The surgical timing of orbital fracture remains controversial. Some investigators support early surgery (within 14 days), and claim complications, such as, diplopia, persistent enophthalmos, and infraorbital hypoesthesia tend to be more common after late than early repair.1, 10, 11, 12 On the other hand, some studies showed successful results for delayed surgery.13, 14, 15, 16, 17, 18 Simon et al13 reported similar outcomes for early (within 2 weeks) and delayed surgery. Other studies showed that good results can be achieved up to 29 days or within 1 month after trauma.14, 15 Belgi et al16 proposed a delayed management protocol to avoid unnecessary surgery for nontrapdoor fracture. Recent study reported orbital floor fracture repair occurring more than 6 weeks or more from injury can achieve marked improvement in both the functional and cosmetic aspects.17 However, delayed surgery may still result in unsatisfactory outcomes, such as, fracture malunion, because of adhesion to adjacent tissues and fibrosis of soft tissues, which can make delayed surgery more technically challenging. In the present study, clinical outcomes including diplopia, enophthalmos, and infraorbital hypoesthesia were similar in early (≤14 days) and delayed (>14 days) surgery groups, and no significant OVR% difference was observed between the two groups immediately after surgery (P=0.891, Table 4). However, the early surgery group showed better mean OVR% at final follow up (P=0.039, Table 4).
During the early posttraumatic period, enophthalmos may not be observed because of orbital soft tissue swelling, but in time it can be observed definitely because of fat necrosis and reduced soft tissue volume. For this reason, during the early posttraumatic period, it is difficult to decide whether surgery is indicated based on the extent of enophthalmos.17, 18 Of the various causes of enophthalmos after blowout fracture, increased orbital volume due to displacement of bony orbit and orbital tissue is considered the most common.2 Bony orbit and herniated orbital tissue volume measurements are useful when deciding whether surgery is indicated and for determining successful orbital wall fracture reconstruction. For this reason, orbital volume has been measured in some studies, but numbers of study subjects recruited were <30, follow ups were <6 months, orbital wall fracture reconstruction rates were not calculated and various aspects, such as, type of orbital implant, fracture location, and time elapsed between trauma and surgical intervention were not taken into account.3, 4, 5, 6, 7, 19 Furthermore, temporal considerations regarding orbital wall fracture reconstruction rates were not investigated because orbital volumes were only measured before and after surgery. Accordingly, in the present study, we decided to measure orbital volumes at three time points (before surgery, immediately after surgery, and at final follow up) using a three-dimensional imaging software program.
The Eclipse Treatment Planning System (ver.13.0, Varian Medical System, Inc.) used in this study was originally used in the radiation and oncology fields to accurately measure organ volumes to determine appropriate dosimetries. When a contour is drawn on orbital CT axial scans, it is drawn on the coronal plane and sagittal plane simultaneously, and when the medium contour differs from that drawn by an observer, the program automatically compensates. This system allows an observer to accurately contour the orbit boundary and minimizes manual errors, and it reconstructs orbital contours as three-dimensional images and calculates orbital volumes automatically.20
In this study, we introduced a new formula to quantify the effect of orbital wall reconstruction by defining a new variable OVR%. In previous studies, the effect of orbital wall reconstruction was evaluated by simply calculating volume differences or volume ratios between fractured and contralateral orbits before and after surgery.3, 4, 5, 6, 7, 19 However, with those calculations, larger differences between the volumes of fractured and contralateral orbits before surgery may result in greater overestimations of the effect of orbital wall reconstruction. To address this limitation, we used OVR%, which provides a measure of how close the volume of the fractured orbit is to the volume of contralateral orbit after surgery, and defined OVR% as  (A=fractured orbit volume after surgery, B=contralateral orbit volume). We measured orbital volumes at three time points (before surgery, immediately after surgery, and at final follow up) and based on these measurements, we were able to calculate OVR% immediately after surgery and at final follow up, and examined relations between OVR% with respect to orbital implant type, orbital wall fracture location, and time elapsed between trauma and surgical intervention.
(A=fractured orbit volume after surgery, B=contralateral orbit volume). We measured orbital volumes at three time points (before surgery, immediately after surgery, and at final follow up) and based on these measurements, we were able to calculate OVR% immediately after surgery and at final follow up, and examined relations between OVR% with respect to orbital implant type, orbital wall fracture location, and time elapsed between trauma and surgical intervention.
In the present study, mean contralateral orbit volume was 21.31±2.50 cm3. Baek et al21 used the Somaris program to measure orbital volume and reported mean normal orbit volumes of 24.51±2.89 cm3 for men and 21.59±2.19 cm3 for women. Kim et al22 used the Silhouette program and reported mean normal orbit volumes of 24.50±2.11 cm3 for men and 23.50±2.09 cm3 for women. Choi et al23 used a semi-automated computer program (MATLAB 2009a) and reported mean normal orbit volumes of 26.48±2.91 cm3 and 23.97± 2.55 cm3 for men and women. In the present study, we did not evaluate orbital volumes by gender and included young patients, who generally have smaller orbits. Importantly, no significant differences were found between measured volumes of contralateral orbits at the three time points (P=0.949), which confirmed the reliability of measured differences between fractured and contralateral orbit volumes. We found that before surgery, mean fractured orbit volume was greater than that of contralateral orbits (P=0.005), and that immediately after surgery, mean fractured orbit volume was significantly smaller than that measured before surgery (P=0.004).
In previous studies on the effect of orbital wall reconstruction based on orbital volume measurements, Fan et al3 reported that 0.89 mm of enophthalmos was improved by a 1 cm3 reduction of fractured orbits in 16 blowout fracture patients using a hydroxyapatite orbital implant. Ploder et al4 reported that 1.2 mm of enophthalmos was improved by a 1 cm3 reduction of fractured orbits in 38 inferior wall fracture patients when orbital volume was measured using region-of-interest measurements taken from CT images. Raskin et al5 reported that 0.47mm of enophthalmos was improved by a 1 cm3 volume reduction of fractured orbits in 30 blowout fracture patients. Fan et al3 reported that 0.89 mm of enophthalmos was improved by a 1 cm3 reduction of fractured orbits in 16 blowout fracture patients using a hydroxyapatite orbital implant. Ye et al6 reported that 0.66 mm enophthalmos was improved by a 1 cm3 fractured orbit reduction in 16 blowout fracture patients using a porous polyethylene orbital implant, and Park et al19 reported that 0.67 mm enophthalmos was improved by a 1 cm3 fractured orbit reduction in 14 blowout fracture patients using an absorbable copolymer mesh orbital implant when orbital volumes were measured using the Rapidia 2.8 program. However, our study failed to find a significant correlation between increased fractured orbit volume and enophthalmos before surgery (P=0.321). Mean enophthalmos before surgery was only 0.4 mm, presumably low level of enophthalmos was due to soft tissue swelling during the early posttraumatic period. Furthermore, mean time elapsed between trauma and surgical intervention was only 7.03 days.
In the present study, the OVR% was 100.47% immediately after surgery and 99.17% at final follow up (P=0.891). In other studies, the time course of reconstruction rate of orbital wall fracture was not addressed because orbital volumes were measured only at two time points (before and after surgery). However, in the present study, orbital volumes were measured before surgery, immediately after surgery, and at final follow up with minimum 6 months(mean 15.2±4.03 months), and thus, we were able to observe that after orbital wall reconstruction orbital volumes were maintained with time when followed up for 15.2±4.03 months. Similarly, no significance intergroup difference was observed between volumes measured immediately after surgery and final follow-up among patients with different fracture locations or patients with different orbital implants. Patients that underwent surgery within 14 days of trauma had a better mean reconstruction rate at final follow up (P=0.039), which supports the advantage of early surgery.1, 10, 11, 12
Summarizing, the effect of orbital wall reconstruction was analyzed quantitatively by measuring orbital volumes using orbital CT then using a new formula ‘Orbital wall reconstruction rate’. For all patients, significant reduction of orbital volume was observed at immediately after orbital wall reconstruction surgery and at final follow up. Using these measurements, factors affecting orbital wall reconstruction were identified. Patients that underwent surgery within 14 days of trauma achieved better reconstruction rates at final follow up, which supports the need for early surgery. There were no significant differences according to orbital implant types or fracture locations. We suggest surgeons bear these findings in mind when planning orbital wall reconstruction. Finally, we found that orbital volume measurements obtained using the described CT-based technique useful for analyzing the effects of orbital wall reconstruction.

References
Jeon SY, Kim C, Ma Y, Hwang E . Microsurgical intranasal reconstruction of blowout fractures of the medial orbital wall. Laryngoscope 1996; 106: 910–913.
Manson PN, Grivas A, Rosenbaum A, Vannier M, Zinreich J, Iliff N . Studies on enophthalmos: II. The measurement of orbital injuries and their treatment by quantitative computed tomography. Plast Reconstr Surg 1986; 77: 203–214.
Fan X, Li J, Zhu J, Li H, Zhang D . Computer-assisted orbital volume measurement in the surgical correction of late enophthalmos caused by blowout fractures. Ophthal Plast Reconstr Surg 2003; 19: 207–211.
Ploder O, Klug C, Voracek M, Burggasser G, Czerny C . Evaluation of computer-based area and volume measurement from coronal computed tomography scans in isolated blowout fractures of the orbital floor. J Oral Maxillofac Surg 2002; 60: 1267–1272.
Raskin EM, Millman AL, Lubkin V, della Rocca RC, Lisman RD, Maher EA . Prediction of late enophthalmos by volumetric analysis of orbital fractures. Ophthal Plast Reconstr Surg 1998; 14: 19–26.
Ye J, Kook KH, Lee SY . Evaluation of computer-based volume measurement and porous polyethylene channel implants in reconstruction of large orbital wall fractures. Invest Ophthalmol Vis Sci 2006; 47: 509–513.
Oh SA, Aum JH, Kang DH, Gu JH . Change of the orbital volume ratio in pure blow-out fractures depending on fracture location. J Craniofac Surg 2013; 24: 1083–1087.
Wilkins RB, Havins WE . Current treatment of blow-out fractures. Ophthalmology 1982; 89: 464–466.
Mathog RH . Management of orbital blow-out fractures. Otolaryngol Clin North Am 1991; 24: 79–91.
Hawes MJ, Dortzbach RK . Surgery on orbital floor fractures. Influence of time of repair and fracture size. Ophthalmology 1983; 90: 1066–1070.
Burnstine MA . Clinical recommendations for repair of isolated orbital floor fractures: an evidence based analysis. Ophthalmology 2002; 109: 1207–1210.
Brucoli M, Arcuri F, Cavenaghi R, Benech A . Analysis of complications after surgical repair of orbital fractures. J Craniofac Surg 2011; 22: 1387–1390.
Simon GJ, Syed HM, McCann JD, Goldberg RA . Early versus late repair of orbital blowout fractures. Ophthalmic Surg Lasers Imaging 2009; 40: 141–148.
Dal Canto AJ, Linberg JV . Comparison of orbital fracture repair performed within 14 days versus 15 to 29 days after trauma. Ophthal Plast Reconstr Surg 2008; 24: 437–443.
Shin KH, Baek SH, Chi M . Comparison of the outcomes of non-trapdoor type blowout fracture repair according to the time of surgery. J Craniofac Surg 2011; 22: 1426–1429.
Beigi B, Khandwala M, Gupta D . Management of pure orbital floor fractures: a proposed protocol to prevent unnecessary or early surgery. Orbit 2014; 33: 336–342.
Scawn RL, Lim LH, Whipple KM, Dolmetsch A, Priel A, Korn B et al. Outcomes of orbital blow-out fracture repair performed beyond 6 weeks after injury. Ophthal Plast Reconstr Surg 2016; 32: 296–301.
Jackson IT . Classification and treatment of orbitozygomatic and orbitoethmoid fractures. The place of bone grafting and plate fixation. Clin Plast Surg 1989; 16: 77–91.
Park YJ, Chung IY, Seo SW . An analysis of orbital reconstruction with bioresorbable plate through orbital volume assessment. J Korean Ophthalmol Soc 2008; 49: 1046–1053.
Mesbahi A, Thwaltes DI, Reilly AJ . Experimental and Monte Carlo evaluation of Eclipse treatment planning system for lung dose calculations. Rep Pract Oncol Radiother 2006; 11: 123–133.
Baek SH, Park MS, Choi JH, Lee TS . Bony orbital volume measurement in normal adults using CT scan. J Korean Ophthalmol Soc 2002; 43: 634–649.
Kim TH, Jun HS, Byun YJ . The normal value of adult korean orbital volume in three-dimensional computerized tomography. J Korean Ophthalmol Soc 2001; 42: 1011–1015.
Choi JH, Park IK, Choi SJ, Shin JH . Measurement of orbital volume from facial CT scans using a semi-automatic computer program. J Korean Ophthalmol Soc 2015; 56: 168–173.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wi, J., Sung, K. & Chi, M. ‘Orbital volume restoration rate after orbital fracture’; a CT-based orbital volume measurement for evaluation of orbital wall reconstructive effect. Eye 31, 713–719 (2017). https://doi.org/10.1038/eye.2016.311
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.311
This article is cited by
-
Subcutaneous emphysema and pneumomediastinum following orbital blowout pathological fracture in a cat with nasal lymphoma: a case report
BMC Veterinary Research (2023)
-
Effect of orbital volume in unilateral orbital fracture on indirect traumatic optic neuropathy
International Ophthalmology (2022)
-
Quantitative assessment of increase in orbital volume after orbital floor fracture reconstruction using a bioabsorbable implant
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)
-
Generation of customized orbital implant templates using 3-dimensional printing for orbital wall reconstruction
Eye (2018)