Abstract
The delayed diagnosis of Duchenne muscular dystrophy (DMD) may be an ongoing problem internationally. We aimed to ascertain age at diagnosis and explore parents’ experiences of the diagnosis of DMD in Australia. Using mixed methods, data were collected from laboratory and clinical record audits of testing for DMD in Victoria and Tasmania, interviews and a national survey of parents regarding their experiences from first noticing symptoms to receiving a diagnosis. The audits revealed that the median age at diagnosis for DMD was 5 years (n=49 during 2005–2010); this age had not changed substantially over this period. Fourteen parents interviewed reported age at diagnosis ranging from 2 to 8 years with a 6 month to 4 year delay between initial concerns about their child’s development and receiving the DMD diagnosis. Sixty-two survey respondents reported the median age at diagnosis was 3 years and 9 months, while the median age when symptoms were noticed was 2 years and 9 months. Parents experienced many emotions in their search for a diagnosis and consulted with a wide range of health professionals. Half the survey respondents felt that their child could have been diagnosed earlier. Despite advances in testing technologies and increasing awareness of DMD, the age at diagnosis has remained constant in Australia. This mixed methods study shows that this diagnostic delay continues to have a negative impact on parents’ experiences, places families at risk of having a second affected child and may have a deleterious effect on affected children’s treatment.
Similar content being viewed by others
Introduction
Duchenne muscular dystrophy (DMD), the most common muscular dystrophy of childhood,1 is degenerative, life-limiting2 and incurable.3 Affected boys have increasing muscle weakness, cardiorespiratory and orthopedic complications,4 and are at a risk of cognitive, behavioral and language difficulties. DMD can initially present with global developmental delay.5 DMD is caused by dystrophin gene variants6 which are inherited from female carriers in approximately two-thirds of cases2 or occur de novo in the remaining one-third,7 although a recent estimate suggests that the incidence of de novo variants may be as high as 50%.8
Historical studies suggest an average age of diagnosis of DMD of ~5 years in the United States and Europe, this figure remaining relatively stable over 26 years.9, 10, 11, 12, 13, 14 Age of diagnosis in Australia has not been previously published. Ciafaloni et al.14 observed a 2.5 year delay between parents first noticing symptoms and ultimately receiving a DMD diagnosis for their child. An earlier diagnosis would mitigate the impact of the diagnostic odyssey on parents9 and facilitate access to a range of health interventions, including corticosteroid treatment and physiotherapy.4 As novel treatments for DMD become available, maximal benefit will be derived from their use early in the disease course.15, 16, 17 Early diagnosis also enables genetic counseling to assist parents in their reproductive planning.14 Despite previous studies reporting diagnostic delay in DMD,9, 11, 14 there are scarce data regarding this process and the associated emotional challenges. An in-depth understanding of parental experiences of the current diagnostic process is essential in determining the role of population screening for DMD. Here we report findings of an Australian study exploring the age at DMD diagnosis through three different data collection methods, and investigating parents’ experiences of the diagnosis of DMD in their child.
Methods
A mixed methods triangulation approach was used in this study, in which interpretation of results are mixed and equal weight is given to both the qualitative and quantitative data.18 We used qualitative data from interviews to develop a quantitative survey, both of which examined parents’ experiences of the process of diagnosis. We further triangulated these data with audit data from Victoria and Tasmania to determine age at DMD diagnosis. The Royal Children’s Hospital (RCH), Melbourne, Human Research Ethics Committee granted ethics approval (HREC30136B).
Audit of DMD testing
Diagnostic testing for DMD in Victoria and Tasmania is performed by statewide laboratories: the Victorian Clinical Genetics Services (VCGS) provides genetic testing and the State Neuropathology Service (SNPS) analyses muscle biopsies. The RCH Neuromuscular Clinic provides multidisciplinary management for Victorian and Tasmanian boys with DMD, and clinicians in the VCGS provide genetic counseling. Information on all DNA tests performed for DMD in these two states between 2005 and 2010 was obtained from VCGS. 2005 was chosen as the audit starting time point because the more sensitive multiplex-ligation probe amplification (MLPA) technique was implemented for DMD in that year (pers. comm., Dr Desiree du Sart, VCGS). Exclusions included tests ordered for: (1) female patients with neuromuscular symptoms (rare for females to have DMD and tests likely requested to exclude DMD); (2) male patients over 10 years old (DMD symptoms usually obvious before 10 years and tests likely ordered to diagnose Becker muscular dystrophy); (3) children previously diagnosed with DMD on muscle biopsies (MLPA likely requested to confirm the diagnosis); and (4) determination of female carrier status for DMD. Inclusion criteria were therefore MLPA tests ordered between 2005 and 2010 for male patients aged ≤10 years showing clinical symptoms of DMD. These MLPA results were then cross-referenced with various databases and patient files: (1) RCH Neuromuscular Clinic patient database and files; (2) VCGS patient files; and (3) SNS database of results of muscle biopsy samples (immunohistochemistry). The following data were collected: (1) total number of DMD diagnoses; (2) MLPA test results (positive/negative); (3) type of testing to obtain DMD diagnosis; (4) age at DMD diagnosis; and (5) medical specialty of ordering physician.
Exploration of parents’ experiences: interviews and survey
Parents to be interviewed were recruited between 2010 and 2011 via an invitation letter through the RCH Neuromuscular Clinic. Inclusion criteria were: parents ≥18 years old, able to speak and read English, with a DMD diagnosis in their child ≥1 year previously. Semi-structured interviews (face-to-face or by telephone), informed by a literature search, enabled questions to be guided by participants’ responses.19 When couples participated, each parent was interviewed separately to explore their individual experience without the partner’s influence, which allowed each parent to freely share views that may have differed from their partner’s. Interviews were digitally recorded and transcribed verbatim. Transcripts were de-identified, participants assigned pseudonyms and transcripts were imported into NVivo 9 software (QSR International Pty Ltd, Melbourne, VIC, Australia) to facilitate data management. Interviews were conducted until no new themes emerged, that is, the data had reached saturation.20
From the qualitative findings, an item bank of questions was generated to inform a survey for distribution to families of boys with DMD throughout Australia. The draft survey was piloted with the original interview participants, who provided feedback regarding: ease with which they could complete the survey; whether the survey items allowed them to provide accurate information to describe their experiences; length, layout and clarity of wording. The final survey included questions on: demographics; number of children in the family with and without DMD and relationship to the responder; initial symptoms of DMD noticed by parents or health professionals; the journey undertaken to find a diagnosis and contacts made with various health professionals; and views on potential changes to the way DMD may be diagnosed in the future. The data presented in this paper relate to the first four categories of questions; views on potential changes in the way DMD may be identified and diagnosed in the future will be presented elsewhere.
In 2012/2013, muscular dystrophy support associations throughout Australia emailed a link for an online version of the survey to the members who were parents of boys with DMD, and mailed out hard-copies when required. Descriptive statistics were used to analyze the data in Microsoft Excel. The age when symptoms were first noticed was determined by ordering the values from youngest to oldest, and taking the middle value as the median.
Results
Description of participants
The audit revealed that 77 boys from Victoria and Tasmania underwent testing. Forty-nine were diagnosed with DMD between 2005 and 2010: 42 were diagnosed based on positive MLPA test results; 5 by muscle biopsy; and 2 because of an affected older brother, clinical findings of DMD and a persistently raised serum creatine kinase. The biopsy results of the five boys were reviewed by our colleagues working at the SNS, and the medical records of the two boys were reviewed by AK to confirm the DMD diagnoses. Interviews were conducted with 14 parents from Victoria (10 mothers, 4 fathers; there were 3 couples); 11 face-to-face and 3 by telephone. Data from the interviews therefore represented the experiences of 14 parents from 11 Victorian families, with two boys in the same family having DMD. Sixty-two anonymous surveys were received from parents nationally. Respondent characteristics are described in Table 1. Not all questions were completed by all respondents.
Age of initial symptoms and at time of diagnosis
The median age at diagnosis for 49 boys as determined by the audit was 5 years (Figure 1a) and was constant over the study period. Approximately one-third (n=16) were diagnosed between two and three years of age; notably, 10 of this group of children (n=16) were diagnosed by a clinician (general pediatrician or pediatric neurologist) in a tertiary hospital (Figure 1b). The median age at diagnosis was 4.5 years when tests were ordered by tertiary pediatricians compared with the median age of 5 years when the tests were ordered by community pediatricians. We conducted statistical analysis (Mann–Whitney) and did not find any significance in this difference, however the numbers are too small to have sufficient power with only 11 tests ordered by community pediatricians.
(a) Audit of clinical and laboratory records: age at DMD diagnosis for all tests ordered 2005–2010 in Victoria and Tasmania (N=49). Median=5 years. (b) Audit of clinical and laboratory records: age at DMD diagnosis for tests ordered by pediatricians and pediatric neurologists (N=42). Median age at diagnosis for tests ordered by paediatricians in tertiary hospitals or paediatric neurologists=4.5 years. Median age at diagnosis for tests ordered by community paediatricians=5 years.
In the interviews, parents (n=14) were asked to recall the ages at diagnosis for their sons. These ages were verified with the audit data, showing a high level of congruence between parent reports and the audit data (Table 2). In most cases there was a delay between the time symptoms were first noticed (ranging from approximately 6 months to 4 years) and the diagnosis of DMD (ranging from 1 year 11 months to 7 years and 11 months, with the median age 3 years and 2 months based on their specific audit data—n=11). In the survey, parents reported that the age at which symptoms were first noticed was a median of 2 years and 8.5 months (n=62), with the median age at diagnosis being 3 years and 8 months (n=62).
Parents’ experiences noticing symptoms and reaching a ‘tipping point’ to seek a diagnosis
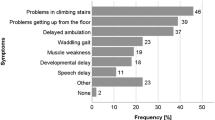
Parents who were interviewed described taking a variety of pathways to obtain the DMD diagnosis, with three main emergent themes shown in Table 2. The transition from noticing symptoms to seeking a diagnosis was described as a period of watching and waiting, in which families described ‘see-sawing’ between reassurance and concern until they ultimately reached a ‘tipping point’. Factors influencing parents’ lack of self-confidence regarding their concerns included being a first-time parent, false reassurance from others, and recognizing the broad range of normal development. On the other hand, those who reached the tipping point sooner were not first-time parents, compared their child with their own other children or those of friends and relatives, and when others noticed symptoms in their child. Symptoms prompting parents to seek medical assessment were examined in the survey and those noted by >20% of parents are shown in Table 3; the most common initial signs were difficulties with running and climbing stairs. Fine motor skills, speech and language, cognitive skills and social function were less often a concern. Many parents discussed this as a time fraught with confusion and emotions:
‘there was a fair amount of stress involved as well, I was feeling low’: Pauline, mother of Caleb
‘you feel so guilty...that you brought these children in the world and...they’ve got this disease to live with’: Hilda, mother of Mark
Many of the feelings reported by the respondents changed during transitions between first noticing symptoms, seeking a diagnosis and after confirmed diagnosis (see Table 3).
Consulting with health professionals regarding concerns about development
In the interviews, parents described in detail their experiences leading up to their child’s diagnosis. For 4 of the 11 families interviewed, the pathway was straightforward; the rest experienced more protracted journeys involving consultations with multiple allied health and/or medical professionals. Fifty-two parents (88%) surveyed reported consulting with a health professional about their concerns when they first noticed symptoms, including: primary care (76%: general practitioners and maternal child health nurses); secondary and tertiary care pediatricians (53%: community pediatricians and hospital-based general pediatricians and pediatric neurologists – NB in Australia a referral is required for this category); allied health professionals (42%: physiotherapists, speech therapists and occupational therapists); complementary health professionals (22%: chiropractors, naturopaths, dieticians and osteopaths). The frequency of consultations with health professionals was also recorded using the categories of ‘once off’, ‘a few times (2–4 times)’ and ‘more often’. Thirty-one percent visited these health professionals ‘more often’ about symptoms in their child.
One aspect that was particularly frustrating for parents who were interviewed was the focus on referrals for early intervention for management of symptoms rather than obtaining a diagnosis, which often came at a later time:
‘...the speech therapist wasn’t doing anything, ‘cause he wasn’t getting any better so (I went) back to my doctor, and she referred me to a pediatric speech person, and he said, ‘he seems to understand everything but it’s just taking time’, and then I went back to my doctor and said that wasn’t good enough, and then she referred me to (early intervention centre)…that was horrible...you just keep getting referred from one place to the next person...it was frustrating.’: Melissa, mother of Stanley
Parents described a tenacious approach to seeking a diagnosis in the face of false reassurances. Of the 52 parents who consulted with a health professional, 83% reported at least one reason for consulting a health professional about their concerns: 46% ‘compared their child with other children who were healthy’; 37% had ‘someone else noticing symptoms’; 29% ‘compared their child with other siblings’ while 25% noticed ‘more or new symptoms’.
Perceptions about the timing of the diagnosis
For all participants, perceptions about the timing of diagnosis were complex. From the survey, views on an earlier diagnosis were mixed: of 57 respondents, 51% indicated that they felt their child could have been diagnosed earlier while 49% did not have this view. A χ2-test was performed to examine whether this response was associated with such variables as parental gender, age, relationship status or education but there were no statistical differences observed, likely because of the small numbers of respondees in each group. Parents in the interviews raised both pros and cons about the possibility of earlier diagnosis.
‘There’s no easy answer because nobody wants to be told at any stage their child has a terminal illness’, Janice, mother of Robert.
Benefits included validation of a reason for the symptoms, ability to access specific treatments sooner, preparing for financial and practical issues in the future, and informing reproductive planning. Parents felt that receiving a diagnosis before symptoms are obvious, however, could be confronting or negatively impact on bonding, and that having to go through a process of noticing symptoms and seeking a diagnosis allows time to gradually accept the diagnosis.
‘…for a year of so [Caleb] was a…normal baby and [we had] normal expectations and, we eventually found out [the diagnosis…I don’t know if I…would have wanted to know…straightaway [at birth].’: Jake, father of Caleb
‘…one of the things about learning at two, with Jeff, was that [we had] a two year old who still wanted to play with his dad…and…those years…will allow a parent to develop acceptance [of the diagnosis].’: Samantha, mother of Jeff
Nevertheless, parents felt it was important that the diagnosis was not too late and that perhaps a time later in infancy or early childhood could be preferable.
Discussion
This study investigates both the age of diagnosis of DMD and parents’ experiences of the process in Australia, and gives a rich perspective of these issues. There continues to be a delay in diagnosis of DMD through standard clinical practice; audit data showed a median age at the diagnosis of 5 years in Victoria and Tasmania, Australia, which has not substantially changed in the last 25 years. International centers report similar findings.9, 10, 11, 12, 13, 14 Parent-reported age at diagnosis was remarkably similar to the actual age at diagnosis, for boys (n=11) of parents who were interviewed, suggesting that parents’ recollections were accurate. However, it should be noted that overall the median age of diagnosis self-reported by the parents in the interview and survey is lower than the age identified by the audit. Given that the self-reported ages from the two groups of parents are more similar, one possibility is that parents who were interviewed or completed the survey represent a biased group as one would expect that the audit data more accurately reflect the time of diagnosis. These parents may also have been more proactive in seeking a diagnosis.
Parents reported consulting a range of different types of health professionals, including complementary health practitioners, when they first started noticing symptoms. The audit data, however, indicated that if boys were referred to pediatricians or pediatric neurologists in tertiary hospitals, testing tended to occur at an earlier age.
Delays in the diagnosis of DMD place families at risk of having a second affected child.11, 14 These delays also affect access to steroid therapy, the only treatment shown to alter the course of DMD, which is usually recommended from the time that motor skills plateau in DMD, typically occurring between the ages of 4 and 6 years. There is increasing evidence that early treatment affects the outcome in DMD,4, 21, 22 and early access to therapies will become increasingly important as novel, more effective treatments become available.15, 17 Other studies have also shown that delays in DMD diagnoses are frustrating and stressful for parents particularly when they are given false reassurance and/or alternate/incorrect diagnoses.9, 23, 24 Indeed, these experiences have been mirrored by parents of children with a range of other genetic conditions including fragile X syndrome (FXS),25 Klinefelter syndrome26 and childhood spinal muscular atrophy (SMA).27
Diagnostic delay in DMD, and other genetic conditions, may occur for several reasons. First, health professionals may not recognize developmental delay given the wide normal range in early childhood.28 The heterogeneity of presentations may slow the recognition of symptoms related to these condition.29 Diagnostic delays may occur due to a lack of awareness and knowledge amongst health professionals, particularly those in the community. Also, children who are initially managed by allied health professionals may not be promptly diagnosed with DMD as allied health professionals (eg, physical, occupational or speech therapists) cannot order screening tests for DMD,14 or because models of care may focus on managing symptoms rather than ascertaining a diagnosis.
Parental factors may also have a role in the timeframe of diagnosis of DMD. During interviews, parents described struggling between feeling that ‘something was wrong’ and feeling that the symptoms were ‘not a big deal’ until they reached a ‘tipping point’ of finally searching for a diagnosis; parents who reach this ‘tipping point’ earlier may receive earlier diagnoses. It is also possible that parents who were assertive and persistent might be more likely to persevere in searching for a cause for their child’s symptoms.
Half the parents in the survey felt that their child could have been diagnosed earlier and, although there was frustration about the delay between noticing signs and ultimately receiving a diagnosis of DMD, parents reported appreciating having that period with their child who appeared normal and healthy before signs of DMD were evident. Strategies to earlier diagnosis could include: raising greater awareness of DMD through continuing professional development and education of health professionals with guidelines that are widely disseminated; screening of children at various ages, such as in infancy (1–2 years of age), and which might be targeted to children showing very early signs of developmental delay; newborn screening, which is currently offered as a clinical service in Antwerp, Belgium and, until late 2011, was available in Wales, United Kingdom.30 Newborn screening for conditions in which there are limited treatment options is controversial due to concerns about the impact a positive screening result may have on the parent–child relationship.31 Welsh data suggest that a DMD diagnosis at birth had not negatively impacted bonding; however, those parents felt that their sons were ‘more precious’ and more likely to describe their sons as ‘cuddly’ compared with parents from the general population.32 Further research is needed to explore options for screening that allow the benefits of earlier identification of DMD and other conditions presenting in early childhood, while minimizing the potential for harms. For some conditions, such as FXS and SMA, carrier screening is also an option, but would be less useful in DMD because of the high rate of sporadic variants.
A number of study limitations must be acknowledged. Assumptions were made to identify patients tested for the purpose of diagnosis of DMD in the audit (ie, excluding records from children >10 years). This may have underestimated the average age at DMD diagnosis. Sample bias may have resulted from only proactive parents and those members of muscular dystrophy support associations participating in the study. Non-members may have differing experiences and opinions. A response rate for this study could not be determined because invitations to join the study were sent by the support associations and the RCH Neuromuscular Clinic as per the requirements of the ethics committee and these groups were unable to provide the number of invited eligible parents.
Conclusion
The age of DMD diagnosis has not significantly decreased internationally nor in Australia. Parents’ experiences and the key factors contributing to the diagnostic delay for families have been described. Clearly strategies need to be put in place to assist in minimizing the diagnostic delay. A variety of approaches, including population-based screening, may be required and evaluated that also consider parental psychosocial impacts.
References
Emery AE : Population frequencies of inherited neuromuscular diseases–a world survey. Neuromuscul Disord 1991; 1: 19–29.
Emery AEH, Emery MLH : The History of a Genetic DIsease: Duchenne Muscular Dystrophy or Meryon's DIsease, 1st edn. London: Royal Society of Medicine Press, 1995.
Muntoni F, Wells D : Genetic treatments in muscular dystrophies. Curr Opin Neurol 2007; 20: 590–594.
Bushby K, Finkel R, Birnkrant DJ et al: Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol 2010; 9: 77–93.
Cyrulnik SE, Fee RJ, De Vivo DC, Goldstein E, Hinton VJ : Delayed developmental language milestones in children with Duchenne's muscular dystrophy. J Pediatr 2007; 150: 474–478.
Murray JM, Davies KE, Harper PS, Meredith L, Mueller CR, Williamson R : Linkage relationship of a cloned DNA sequence on the short arm of the X chromosome to Duchenne muscular dystrophy. Nature 1982; 300: 69–71.
Emery AEH (ed): Duchenne Muscular Dystrophy, 2nd edn. Oxford, New York: Oxford University Press, 1987.
Helderman-van den Enden ATJM, Madan K, Breuning MH et al: An urgent need for a change in policy revealed by a study on prenatal testing for Duchenne muscular dystrophy. Eur J Hum Genet 2013; 21: 21–26.
Firth M, Gardner-Medwin D, Hosking G, Wilkinson E : Interviews with parents of boys suffering from Duchenne muscular dystrophy. Dev Med Child Neurol 1983; 25: 466–471.
Read L, Galasko CS : Delay in diagnosing Duchenne muscular dystrophy in orthopaedic clinics. J Bone Joint Surg Br 1986; 68: 481–482.
Bushby KM, Hill A, Steele JG : Failure of early diagnosis in symptomatic Duchenne muscular dystrophy. Lancet 1999; 353: 557–558.
Zalaudek I, Bonelli RM, Koltringer P, Reisecker F, Wagner K : Early diagnosis in Duchenne muscular dystrophy. Lancet 1999; 353: 1975.
Mohamed K, Appleton R, Nicolaides P : Delayed diagnosis of Duchenne muscular dystrophy. Eur J Paediatr Neurol 2000; 4: 219–223.
Ciafaloni E, Fox DJ, Pandya S et al: Delayed diagnosis in duchenne muscular dystrophy: data from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). J Pediatr 2009; 155: 380–385.
Laing NG : Multiplicity of experimental approaches to therapy for genetic muscle diseases and necessity for population screening. J Muscle Res Cell Motil 2008; 29: 247–252.
Wells DJ : Therapeutic restoration of dystrophin expression in Duchenne muscular dystrophy. J Muscle Res Cell Motil 2006; 27: 387–398.
Kakulas BA : Problems and potential for gene therapy in Duchenne muscular dystrophy. Neuromuscul Disord 1997; 7: 319–324.
Creswell JW, Plano Clark VL : Designing and Conducting Mixed Methods Research, 2nd edn. Thousand Oaks: Sage Publications, 2007.
Hansen EC : Successful qualitative health research: a practical introduction. NSW, Australia: Allen & Urwin, 2006.
Corbin J, Strauss A : Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory, 3rd edn. Thousand Oaks: Sage, 2007.
McDonald CM, Han JJ, Mah JK, Carter GT : Corticosteroids and Duchenne muscular dystrophy: does earlier treatment really matter? Muscle Nerve 2012; 45: 777–779.
Ricotti V, Ridout DA, Scott E et al: Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatr 2013; 84: 698–705.
Firth MA : Diagnosis of Duchenne muscular dystrophy: experiences of parents of sufferers. Br Med J 1983; 286: 700–701.
Eilers R, Kleinveld JH, Vroom E, Westerman MJ, Cornel MC, Plass AMC : Desirability of early identification of Duchenne Muscular Dystrophy (DMD): parents' experiences of the period prior to diagnosis. Eur J Hum Genet 2010;18 (Supp 1): 373–374.
Bailey DB, Skinner D, Hatton D, Roberts J : Family experiences and factors associated with the diagnosis of fragile X syndrome. J Dev Behav Pediatr 2000; 21: 315–321.
Bourke E, Snow P, Herlihy A, Amor D, Metcalfe S : A qualitative exporation of mothers’ and fathers’ experiences of having a child with Klinefelter syndrome and the process of reaching this diagnosis. Eur J Hum Genet 2014; 22: 18–24.
Lawton S, Hickerton C, Archibald AD, McClaren BJ, Metcalfe SA : A mixed methods exploration of families’ experiences of the diagnosis of childhood spinal muscular atrophy. Eur J Hum Genet 2014, ; e-pub ahead of print 30 July 2014 doi:10.1038/ejhg.2014.147.
Bellman M, Byrne O, Sege R : Developmental assessment of children. Br Med J 2013; 346: e8687.
Parsons EP, Clarke AJ, Bradley DM : Developmental progress in Duchenne muscular dystrophy: lessons for earlier detection. Eur J Paediatr Neurol 2004; 8: 145–153.
Mendell JR, Shilling C, Leslie ND et al: Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann Neurol 2012; 71: 304–313.
Ross LF : Screening for conditions that do not meet the Wilson and Jungner criteria: the case of Duchenne muscular dystrophy. Am J Med Genet 2006; 140A: 914–922.
Parsons EP, Clarke AJ, Hood K, Lycett E, Bradley DM : Newborn screening for Duchenne muscular dystrophy: a psychosocial study. Arch Dis Child Fetal Neonatal Ed 2002; 86: F91–F95.
Acknowledgements
We are grateful to participating families, to Daniella Villano and Anita Mach of the RCH Neuromuscular Clinic for distributing invitation letters and for helping with the clinical audit. We thank the following for distributing the survey to their members: Muscular Dystrophy Australia; Muscular Dystrophy New South Wales; Muscular Dystrophy Tasmania; Muscular Dystrophy Western Australia; Duchenne Foundation and Muscular Dystrophy South Australia. We thank Boris Struk for his encouragement and support of the study and to the Victorian Government’s Infrastructure Support Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wong, S., McClaren, B., Archibald, A. et al. A mixed methods study of age at diagnosis and diagnostic odyssey for Duchenne muscular dystrophy. Eur J Hum Genet 23, 1294–1300 (2015). https://doi.org/10.1038/ejhg.2014.301
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2014.301
This article is cited by
-
Time to diagnosis of Duchenne muscular dystrophy in Austria and Germany
Scientific Reports (2023)
-
Mutational spectrum and phenotypic variability of Duchenne muscular dystrophy and related disorders in a Bangladeshi population
Scientific Reports (2023)
-
Genotype characterization and delayed loss of ambulation by glucocorticoids in a large cohort of patients with Duchenne muscular dystrophy
Orphanet Journal of Rare Diseases (2021)
-
A review of quality of life themes in Duchenne muscular dystrophy for patients and carers
Health and Quality of Life Outcomes (2018)
-
Clinical management of Duchenne muscular dystrophy: the state of the art
Neurological Sciences (2018)