Abstract
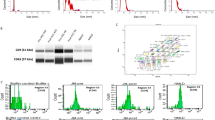
Pathological confirmation is desired prior to high-risk surgery for suspected perihilar cholangiocarcinoma (PHC), but preoperative tissue diagnosis is limited by poor sensitivity of available techniques. This study aimed to validate whether a tumor-specific enhanced green fluorescent protein (eGFP)-expressing oncolytic virus could be used for cholangiocarcinoma (CC) cell detection. Extrahepatic CC cell lines SK-ChA-1, EGI-1, TFK-1 and control cells (primary human liver cells) were exposed to the oncolytic herpes simplex type 1 virus NV1066 for up to 24 h in adherent culture. The technique was validated for cells in suspension and cultured cells that had been exposed to crude patient bile. Optimal incubation time of the CC cells with NV1066 at a multiplicity of infection of 0.1 was determined at 6–8 h, yielding 15% eGFP-expressing cells, as measured by flow cytometry. Cells were able to survive 2-h crude bile exposure and remained capable of producing eGFP following NV1066 infection. Detection of malignant cells was possible at the highest dilution tested (10 CC cells among 2 × 105 control cells), though hampered by non-target cell autofluorescence. The technique was not applicable to cells in suspension due to insufficient eGFP production. Accordingly, as yet the technique is not suitable for standardized clinical diagnostics in PHC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996; 224: 463–473; discussion 473-5.
Ruys AT, van Haelst S, Busch OR, Rauws EA, Gouma DJ, van Gulik TM . Long-term survival in hilar cholangiocarcinoma also possible in unresectable patients. World J Surg 2012; 36: 2179–2186.
Coelen RJ, Wiggers JK, Nio CY, Besselink MG, Busch OR, Gouma DJ et al. Preoperative computed tomography assessment of skeletal muscle mass is valuable in predicting outcomes following hepatectomy for perihilar cholangiocarcinoma. HPB (Oxford) 2015; 17: 520–528.
van Gulik TM, Kloek JJ, Ruys AT, Busch OR, van Tienhoven GJ, Lameris JS et al. Multidisciplinary management of hilar cholangiocarcinoma (Klatskin tumor): extended resection is associated with improved survival. Eur J Surg Oncol 2011; 37: 65–71.
Gerhards MF, Vos P, van Gulik TM, Rauws EA, Bosma A, Gouma DJ . Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br J Surg 2001; 88: 48–51.
Tamada K, Ushio J, Sugano K . Endoscopic diagnosis of extrahepatic bile duct carcinoma: Advances and current limitations. World J Clin Oncol 2011; 2: 203–216.
Hattori M, Nagino M, Ebata T, Kato K, Okada K, Shimoyama Y . Prospective study of biliary cytology in suspected perihilar cholangiocarcinoma. Br J Surg 2011; 98: 704–709.
Kloek JJ, van Delden OM, Erdogan D, ten Kate FJ, Rauws EA, Busch OR et al. Differentiation of malignant and benign proximal bile duct strictures: the diagnostic dilemma. World J Gastroenterol 2008; 14: 5032–5038.
Mohamadnejad M, DeWitt JM, Sherman S, LeBlanc JK, Pitt HA, House MG et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: a large single-center experience. Gastrointest Endosc 2011; 73: 71–78.
Williamson JB, Draganov PV . The usefulness of SpyGlass choledochoscopy in the diagnosis and treatment of biliary disorders. Curr Gastroenterol Rep 2012; 14: 534–541.
Tringali A, Lemmers A, Meves V, Terheggen G, Pohl J, Manfredi G et al. Intraductal biliopancreatic imaging: European Society of Gastrointestinal Endoscopy (ESGE) technology review. Endoscopy 2015; 47: 739–753.
Navaneethan U, Njei B, Venkatesh PG, Lourdusamy V, Sanaka MR . Endoscopic ultrasound in the diagnosis of cholangiocarcinoma as the etiology of biliary strictures: a systematic review and meta-analysis. Gastroenterol Rep (Oxf) 2015; 3: 209–215.
Navaneethan U, Hasan MK, Lourdusamy V, Njei B, Varadarajulu S, Hawes RH . Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc 2015; 82: 608–14.e2.
Wong RJ, Joe JK, Kim SH, Shah JP, Horsburgh B, Fong Y . Oncolytic herpesvirus effectively treats murine squamous cell carcinoma and spreads by natural lymphatics to treat sites of lymphatic metastases. Hum Gene Ther 2002; 13: 1213–1223.
Chiocca EA . Oncolytic viruses. Nat Rev Cancer 2002; 2: 938–950.
Fong SM, Lee MK, Adusumilli PS, Kelly KJ . Fluorescence-expressing viruses allow rapid identification and separation of rare tumor cells in spiked samples of human whole blood. Surgery 2009; 146: 498–505.
Adusumilli PS, Gholami S, Chun YS, Mullerad M, Chan MK, Yu Z et al. Fluorescence-assisted cytological testing (FACT): Ex Vivo viral method for enhancing detection of rare cancer cells in body fluids. Mol Med 2011; 17: 628–634.
Holman HA, MacLean AR . Neurovirulent factor ICP34.5 uniquely expressed in the herpes simplex virus type 1 Delta gamma 1 34.5 mutant 1716. J Neurovirol 2008; 14: 28–40.
Israyelyan A, Chouljenko VN, Baghian A, David AT, Kearney MT, Kousoulas KG . Herpes simplex virus type-1(HSV-1) oncolytic and highly fusogenic mutants carrying the NV1020 genomic deletion effectively inhibit primary and metastatic tumors in mice. Virol J 2008; 5: 68.
Knuth A, Gabbert H, Dippold W, Klein O, Sachsse W, Bitter-Suermann D et al. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol 1985; 1: 579–596.
Saijyo S, Kudo T, Suzuki M, Katayose Y, Shinoda M, Muto T et al. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med 1995; 177: 61–71.
Scherdin G, Garbrecht M, Kouche M . In vitro interaction of α-difluoromethyl-ornithine (DFMO) and human recombinant interferon-γ (rIFN-γ) on human cancer cell lines. Immunobiology 1987; 175: 1–431, abstract B.21.
van 't Wout AB, Schuitemaker H, Kootstra NA . Isolation and propagation of HIV-1 on peripheral blood mononuclear cells. Nat Protoc 2008; 3: 363–370.
Vichai V, Kirtikara K . Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 2006; 1: 1112–1116.
Kusser KL, Randall TD . Simultaneous detection of EGFP and cell surface markers by fluorescence microscopy in lymphoid tissues. J Histochem Cytochem 2003; 51: 5–14.
Kelly KJ, Wong J, Gonen M, Allen P, Brennan M, Coit D et al. Human trial of a genetically modified herpes simplex virus for rapid detection of positive peritoneal cytology in the staging of pancreatic cancer. EBioMedicine 2016; 7: 94–99.
Ogle LF, Orr JG, Willoughby CE, Hutton C, McPherson S, Plummer R et al. Imagestream detection and characterisation of circulating tumour cells - A liquid biopsy for hepatocellular carcinoma? J Hepatol 2016; 65: 305–313.
Jarnagin WR, Zager JS, Hezel M, Stanziale SF, Adusumilli PS, Gonen M et al. Treatment of cholangiocarcinoma with oncolytic herpes simplex virus combined with external beam radiation therapy. Cancer Gene Ther 2006; 13: 326–334.
Pugalenthi A, Mojica K, Ady JW, Johnsen C, Love D, Chen NG et al. Recombinant vaccinia virus GLV-1h68 is a promising oncolytic vector in the treatment of cholangiocarcinoma. Cancer Gene Ther 2015; 22: 591–596.
Eisenberg DP, Carpenter SG, Adusumilli PS, Chan MK, Hendershott KJ, Yu Z et al. Hyperthermia potentiates oncolytic herpes viral killing of pancreatic cancer through a heat shock protein pathway. Surgery 2010; 148: 325–334.
Ady JW, Johnsen C, Mojica K, Heffner J, Love D, Pugalenthi A et al. Oncolytic gene therapy with recombinant vaccinia strain GLV-2b372 efficiently kills hepatocellular carcinoma. Surgery 2015; 158: 331–338.
Ady JW, Heffner J, Mojica K, Johnsen C, Belin LJ, Love D et al. Oncolytic immunotherapy using recombinant vaccinia virus GLV-1h68 kills sorafenib-resistant hepatocellular carcinoma efficiently. Surgery 2014; 156: 263–269.
Ramchandani M, Reddy DN, Gupta R, Lakhtakia S, Tandan M, Darisetty S et al. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc 2011; 74: 511–519.
Siddiqui AA, Mehendiratta V, Jackson W, Loren DE, Kowalski TE, Eloubeidi MA . Identification of cholangiocarcinoma by using the Spyglass Spyscope system for peroral cholangioscopy and biopsy collection. Clin Gastroenterol Hepatol 2012; 10: 466–471; quiz e48.
Doorenspleet ME, Hubers LM, Culver EL, Maillette de Buy Wenniger LJ, Klarenbeek PL, Chapman RW et al. IgG4+ B-cell receptor clones distinguish IgG4-related disease from primary sclerosing cholangitis and biliary/pancreatic malignancies. Hepatology 2016; 64: 501–507.
Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014; 6: 224ra24.
Acknowledgements
We are grateful to Dr NA Kootstra and A van Nuenen for providing facility for viral culturing. We thank B Hooijbrink for assisting in the flow cytometry analysis and Prof. S Repping for providing the Vero cells. The SK-ChA-1 cells were a kind gift from A Knuth and C Matter from the University Hospital Zurich, Switzerland. The EGI-1 and TFK-1 cells were provided by Prof. RP Oude Elferink from the Tytgat Institute for Liver and Intestinal Research, Department of Gastroenterology and Hepatology, Academic Medical Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This study was previously presented at the 51st Congress of the European Society for Surgical Research, 25–28 May 2016, Prague, Czech Republic.
Supplementary Information accompanies the paper on Cancer Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Coelen, R., de Keijzer, M., Weijer, R. et al. In vitro detection of cholangiocarcinoma cells using a fluorescent protein-expressing oncolytic herpes virus. Cancer Gene Ther 24, 227–232 (2017). https://doi.org/10.1038/cgt.2017.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2017.11