Abstract
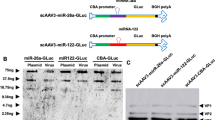
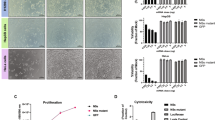
Precise oncotropism is required for successful systemic administration of next-generation oncolytic measles viruses (MVs). We have previously established a system for efficient post-entry targeting by insertion of synthetic microRNA target sites (miRTS) into the MV genome, thereby repressing replication in the presence of cognate microRNAs. Thus, differential expression of microRNAs, as frequently observed in normal compared with malignant tissues, can be exploited to increase vector specificity and safety. Here we report the combination of miRTS for different microRNAs in a single vector to detarget pivotal organs at risk during systemic administration (liver, brain, gastrointestinal tract). Accordingly, miRTS for miR-122, miR-7 and miR-148a that are enriched in these tissues were inserted to create multi-tissue-detargeted MV (MV-EGFPmtd). Replication of MV-EGFPmtd is repressed in cell lines as well as in non-transformed primary human hepatocytes and liver slices expressing cognate microRNAs. Oncolytic potency of MV-EGFPmtd is retained in a model of pancreatic cancer in vitro and in vivo. This work is a proof-of-concept that favorable expression profiles of multiple microRNAs can be exploited concomitantly to reshape the tropism of MV without compromising oncolytic efficacy. This strategy can be adapted to different vectors and cancer entities for safe and efficient high-dose systemic administration in clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Galanis E, Hartmann L, Cliby W, Long H, Peethambaram P, Barrette B et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res 2010; 70: 875–882.
Russell SJ, Federspiel MJ, Peng K, Tong C, Dingli D, Morice WG et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc 2014; 89 (7): 926–933.
Esolen LM, Park SW, Hardwick JM, Griffin DE . Apoptosis as a cause of death in measles virus-infected cells. J Virol 1995; 69: 3955–3958.
Miest TS, Cattaneo R . New viruses for cancer therapy: meeting clinical needs. Nat Rev Microbiol 2014; 12: 23–34.
Bossow S, Grossardt C, Temme A, Leber MF, Sawall S, Rieber EP et al. Armed and targeted measles virus for chemovirotherapy of pancreatic cancer. Cancer Gene Ther 2011; 18 (8): 598–608.
Grossardt C, Engeland CE, Bossow S, Halama N, Zaoui K, Leber MF et al. Granulocyte-macrophage colony-stimulating factor-armed oncolytic measles virus is an effective therapeutic cancer vaccine. Hum Gene Ther 2013; 24: 644–654.
Leber M, Bossow S, Leonard V, Zaoui K, Grossardt C, Frenzke M et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol Ther 2011; 19: 1097–1106.
Ungerechts G, Frenzke ME, Yaiw K, Miest T, Johnston PB, Cattaneo R . Mantle cell lymphoma salvage regimen: synergy between a reprogrammed oncolytic virus and two chemotherapeutics. Gene Therapy 2010; 17: 1506–1516.
Miest T, Yaiw K, Frenzke M, Lampe J, Hudacek A, Springfeld C et al. Envelope-chimeric entry-targeted measles virus escapes neutralization and achieves oncolysis. Mol Ther 2011; 19: 1813–1820.
Kaufmann JK, Bossow S, Grossardt C, Sawall S, Kupsch J, Erbs P et al. Chemovirotherapy of malignant melanoma with a targeted and armed oncolytic measles virus. J Invest Dermatol 2013; 133: 1034–1042.
Navaratnarajah CK, Miest TS, Carfi A, Cattaneo R . Targeted entry of enveloped viruses: measles and herpes simplex virus I. Curr Opin Virol 2012; 2: 43–49.
Ungerechts G, Springfeld C, Frenzke M, Lampe J, Johnston P, Parker W et al. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res 2007; 67: 10939–10947.
Ungerechts G, Springfeld C, Frenzke M, Lampe J, Parker W, Sorscher E et al. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol Ther 2007; 15: 1991–1997.
Friedrich K, Hanauer JR, Prufer S, Munch RC, Volker I, Filippis C et al. DARPin-targeting of measles virus: unique bispecificity, effective oncolysis, and enhanced safety. Mol Ther 2013; 21: 849–859.
Hudacek AW, Navaratnarajah CK, Cattaneo R . Development of measles virus-based shielded oncolytic vectors: suitability of other paramyxovirus glycoproteins. Cancer Gene Ther 2013; 20: 109–116.
Edge RE, Falls TJ, Brown CW, Lichty BD, Atkins H, Bell JC . A let-7 MicroRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol Ther 2008; 16: 1437–1443.
Kelly E, Hadac E, Greiner S, Russell S . Engineering microRNA responsiveness to decrease virus pathogenicity. Nat Med 2008; 14: 1278–1283.
Kelly EJ, Nace R, Barber GN, Russell SJ . Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting. J Virol 2010; 84: 1550–1562.
Lee Cleo YF, Rennie PS, Jia WW . MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells. Clin Cancer Res 2009; 15: 5126–5135.
Ylosmaki E, Hakkarainen T, Hemminki A, Visakorpi T, Andino R, Saksela K . Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA. J Virol 2008; 82: 11009–11015.
Myers RM, Greiner SM, Harvey ME, Griesmann G, Kuffel MJ, Buhrow SA et al. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin Pharmacol Ther 2007; 82: 700–710.
Chang J, Nicolas E, Marks D, Sander C, Lerro A, Buendia M et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol 2004; 1: 106–113.
Kutay H, Bai S, Datta J, Motiwala T, Pogribny I, Frankel W et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem 2006; 99: 671–678.
Bai S, Nasser M, Wang B, Hsu S, Datta J, Kutay H et al. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem 2009; 284: 32015–32027.
Fu X, Rivera A, Tao L, De G, Zhang X . Construction of an oncolytic herpes simplex virus that precisely targets hepatocellular carcinoma cells. Mol Ther 2011; 20 (2): 339–346.
Ylosmaki E, Lavilla-Alonso S, Jaamaa S, Vaha-Koskela M, af Hallstrom T, Hemminki A et al. MicroRNA-mediated suppression of oncolytic adenovirus replication in human liver. PLoS One 2013; 8: e54506.
Szafranska AE, Davison TS, John J, Cannon T, Sipos B, Maghnouj A et al. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene 2007; 26: 4442–4452.
Bloomston M, Frankel W, Petrocca F, Volinia S, Alder H, Hagan J et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA 2007; 297: 1901–1908.
Mestdagh P, Lefever S, Pattyn F, Ridzon D, Fredlund E, Fieuw A et al. The microRNA body map: dissecting microRNA function through integrative genomics. Nucleic Acids Res 2011; 39: e136.
Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C et al. Rescue of measles viruses from cloned DNA. EMBO J 1995; 14: 5773–5784.
Muhlebach MD, Schaser T, Zimmermann M, Armeanu S, Hanschmann KO, Cattaneo R et al. Liver cancer protease activity profiles support therapeutic options with matrix metalloproteinase-activatable oncolytic measles virus. Cancer Res 2010; 70: 7620–7629.
Zimmermann M, Weiland T, Bitzer M, Lauer UM . Preclinical testing of virotherapeutics for primary and secondary tumors of the liver. Methods Mol Biol 2012; 806: 121–136.
Rima BK, Duprex WP . The measles virus replication cycle. Curr Top Microbiol Immunol 2009; 329: 77–102.
Huntzinger E, Izaurralde E . Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 2011; 12: 99–110.
Wild TF, Buckland R . Functional aspects of envelope-associated measles virus proteins. Curr Top Microbiol Immunol 1995; 191: 51–64.
Wild TF, Malvoisin E, Buckland R . Measles virus: both the haemagglutinin and fusion glycoproteins are required for fusion. J Gen Virol 1991; 72 (Pt 2): 439–442.
Russell SJ, Peng K, Bell JC . Oncolytic virotherapy. Nat Biotechnol 2012; 30: 658–670.
Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007; 129: 1401–1414.
Hsu S, Chu C, Tsou A, Chen S, Chen H, Hsu PW et al. miRNAMap 2.0: genomic maps of microRNAs in metazoan genomes. Nucleic Acids Res 2008; 36: D165–D169.
Sugio K, Sakurai F, Katayama K, Tashiro K, Matsui H, Kawabata K et al. Enhanced safety profiles of the telomerase-specific replication-competent adenovirus by incorporation of normal cell-specific microRNA-targeted sequences. Clin Cancer Res 2011; 17: 2807–2818.
Negrini M, Gramantieri L, Sabbioni S, Croce CM . microRNA involvement in hepatocellular carcinoma. Anticancer Agents Med Chem 2011; 11: 500–521.
Acknowledgements
We thank Silke Hamzaoui-Nord and Claudia Lay-Mees for their animal care support, Birgit Hoyler for technical support, Michael Mühlebach for the anti-F/-H antibodies, Roberto Cattaneo for his continuous support and the anti-N antibody, Jürgen Weitz and Hubertus Schmitz-Winnenthal for the primary liver material and Frank Bergmann for pathological analyses. This work was supported by Deutsche Krebshilfe (German Cancer Aid), Max Eder Program No. 110702 (to GU), DKFZ Heinrich F. C. Behr stipends (to MAB and MS) and a Helmholtz-Association/DKFZ PhD fellowship (to MFL). This work was done in Heidelberg, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Baertsch, M., Leber, M., Bossow, S. et al. MicroRNA-mediated multi-tissue detargeting of oncolytic measles virus. Cancer Gene Ther 21, 373–380 (2014). https://doi.org/10.1038/cgt.2014.40
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2014.40
This article is cited by
-
Onkolytische Viren zur Behandlung von Krebserkrankungen
BIOspektrum (2023)
-
Regulatory Effects and Mechanism of Adenovirus-Mediated PTEN Gene on Hepatic Stellate Cells
Digestive Diseases and Sciences (2016)
-
MicroRNA-7: a promising new target in cancer therapy
Cancer Cell International (2015)
-
Oncolysis by paramyxoviruses: preclinical and clinical studies
Molecular Therapy - Oncolytics (2015)