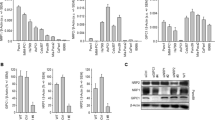
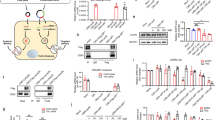
Abstract
RNA interference (RNAi) represents a powerful, new tool for scientific investigation as well as a promising new form of targeted gene therapy, with applications currently in clinical trials. Bifunctional short hairpin RNA (shRNA) are synthetic RNAi molecules, engineered to utilize multiple endogenous RNAi pathways to specifically silence target genes. Pancreatic and duodenal homeobox 1 (PDX1) is a key regulator of pancreatic development, β-cell differentiation, normal β-cell function and pancreatic cancer. Our aim is to review the process of identifying PDX1 as a specific, potential RNAi target in pancreatic cancer, as well as the underlying mechanisms and various forms of RNAi, with subsequent testing and development of PDX1-targeted bifunctional shRNA therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Howlader NNA, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL (eds) SEER Cancer Statistics Review 1975-2010. National Cancer Institute: Bethesda, MD, 2013.
Cowgill SM, Muscarella P . The genetics of pancreatic cancer. Am J Surg 2003; 186: 279–286.
Hong SM, Park JY, Hruban RH, Goggins M . Molecular signatures of pancreatic cancer. Arch Pathol Lab Med 2011; 135: 716–727.
Liu SH, Patel S, Gingras MC, Nemunaitis J, Zhou G, Chen C et al. PDX-1: demonstration of oncogenic properties in pancreatic cancer. Cancer 2011; 117: 723–733.
Liu SH, Rao DD, Nemunaitis J, Senzer N, Zhou G, Dawson D et al. PDX-1 is a therapeutic target for pancreatic cancer, insulinoma and islet neoplasia using a novel RNA interference platform. PLoS One 2012; 7: e40452.
Liu T, Gou SM, Wang CY, Wu HS, Xiong JX, Zhou F . Pancreas duodenal homeobox-1 expression and significance in pancreatic cancer. World J Gastroenterol 2007; 13: 2615–2618.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T . Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411: 494–498.
Habener JF, Kemp DM, Thomas MK . Minireview: transcriptional regulation in pancreatic development. Endocrinology 2005; 146: 1025–1034.
Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 1996; 122: 983–995.
Habener JF, Stoffers DA . A newly discovered role of transcription factors involved in pancreas development and the pathogenesis of diabetes mellitus. Proc Assoc Am Physicians 1998; 110: 12–21.
Holland AM, Gonez LJ, Naselli G, Macdonald RJ, Harrison LC . Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes 2005; 54: 2586–2595.
Macfarlane WM, Frayling TM, Ellard S, Evans JC, Allen LI, Bulman MP et al. Missense mutations in the insulin promoter factor-1 gene predispose to type 2 diabetes. J Clin Invest 1999; 104: R33–R39.
Hani EH, Stoffers DA, Chevre JC, Durand E, Stanojevic V, Dina C et al. Defective mutations in the insulin promoter factor-1 (IPF-1) gene in late-onset type 2 diabetes mellitus. J Clin Invest 1999; 104: R41–R48.
Shiau MY, Huang CN, Liao JH, Chang YH . Missense mutations in the human insulin promoter factor-1 gene are not a common cause of type 2 diabetes mellitus in Taiwan. J Endocrinol Invest 2004; 27: 1076–1080.
Banakh I, Gonez LJ, Sutherland RM, Naselli G, Harrison LC . Adult pancreas side population cells expand after β cell injury and are a source of insulin-secreting cells. PLoS One 2012; 7: e48977.
Li W-C, Rukstalis JM, Nishimura W, Tchipashvili V, Habener JF, Sharma A et al. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J Cell Sci 2010; 123 (Pt 16): 2792–2802.
Strobel O, Rosow DE, Rakhlin EY, Lauwers GY, Trainor AG, Alsina J et al. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology 2010; 138: 1166–1177.
Szabat M, Luciani DS, Piret JM, Johnson JD . Maturation of adult beta-cells revealed using a Pdx1/insulin dual-reporter lentivirus. Endocrinology 2009; 150: 1627–1635.
Yoneda S, Uno S, Iwahashi H, Fujita Y, Yoshikawa A, Kozawa J et al. Predominance of beta-cell neogenesis rather than replication in humans with an impaired glucose tolerance and newly diagnosed diabetes. J Clin Endocrinol Metab 2013; 98: 2053–2061.
Koya V, Lu S, Sun YP, Purich DL, Atkinson MA, Li SW et al. Reversal of streptozotocin-induced diabetes in mice by cellular transduction with recombinant pancreatic transcription factor pancreatic duodenal homeobox-1: a novel protein transduction domain-based therapy. Diabetes 2008; 57: 757–769.
Madsen OD, Jensen J, Petersen HV, Pedersen EE, Oster A, Andersen FG et al. Transcription factors contributing to the pancreatic beta-cell phenotype. Horm Metab Res 1997; 29: 265–270.
Serup P, Jensen J, Andersen FG, Jorgensen MC, Blume N, Holst JJ et al. Induction of insulin and islet amyloid polypeptide production in pancreatic islet glucagonoma cells by insulin promoter factor 1. Proc Natl Acad Sci USA 1996; 93: 9015–9020.
Abraham EJ, Leech CA, Lin JC, Zulewski H, Habener JF . Insulinotropic hormone glucagon-like peptide-1 differentiation of human pancreatic islet-derived progenitor cells into insulin-producing cells. Endocrinology 2002; 143: 3152–3161.
Tirone TA, Fagan SP, Templeton NS, Wang X, Brunicardi FC . Insulinoma-induced hypoglycemic death in mice is prevented with beta cell-specific gene therapy. Ann Surg 2001; 233: 603–611.
Wang XP, Li ZJ, Magnusson J, Brunicardi FC . Tissue MicroArray analyses of pancreatic duodenal homeobox-1 in human cancers. World J Surg 2005; 29: 334–338.
Koizumi M, Doi R, Toyoda E, Masui T, Tulachan SS, Kawaguchi Y et al. Increased PDX-1 expression is associated with outcome in patients with pancreatic cancer. Surgery 2003; 134: 260–266.
Quint K, Stintzing S, Alinger B, Hauser-Kronberger C, Dietze O, Gahr S et al. The expression pattern of PDX-1, SHH, Patched and Gli-1 is associated with pathological and clinical features in human pancreatic cancer. Pancreatology 2009; 9: 116–126.
Smit VT, Boot AJ, Smits AM, Fleuren GJ, Cornelisse CJ, Bos JL . KRAS codon 12 mutations occur very frequently in pancreatic adenocarcinomas. Nucleic Acids Res 1988; 16: 7773–7782.
Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M . Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 1988; 53: 549–554.
Gidekel Friedlander SY, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D et al. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell 2009; 16: 379–389.
Levine L, Wy KY, Herrmann H . Decreased levels of an inhibitor of prostaglandin E 9-ketoreductase activity in chick dystrophic breast muscle. Nature 1976; 260: 791–793.
Aigner A . Delivery systems for the direct application of siRNAs to induce RNA interference (RNAi) in vivo. J Biomed Biotechnol 2006; 2006: 71659.
Love TM, Moffett HF, Novina CD . Not miR-ly small RNAs: big potential for microRNAs in therapy. J Allergy Clin Immunol 2008; 121: 309–319.
Senior JH . Fate and behavior of liposomes in vivo: a review of controlling factors. Crit Rev Ther Drug Carrier Syst 1987; 3: 123–193.
Pan Q, Ramakrishnaiah V, Henry S, Fouraschen S, de Ruiter PE, Kwekkeboom J et al. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi). Gut 2012; 61: 1330–1339.
Chen LM, Le HY, Qin RY, Kumar M, Du ZY, Xia RJ et al. Reversal of the phenotype by K-rasval12 silencing mediated by adenovirus-delivered siRNA in human pancreatic cancer cell line Panc-1. World J Gastroenterol 2005; 11: 831–838.
Fleming JB, Shen GL, Holloway SE, Davis M, Brekken RA . Molecular consequences of silencing mutant K-ras in pancreatic cancer cells: justification for K-ras-directed therapy. Mol Cancer Res 2005; 3: 413–423.
Wang W, Wang CY, Dong JH, Chen X, Zhang M, Zhao G . Identification of effective siRNA against K-ras in human pancreatic cancer cell line MiaPaCa-2 by siRNA expression cassette. World J Gastroenterol 2005; 11: 2026–2031.
Senzer N, Barve M, Kuhn J, Melnyk A, Beitsch P, Lazar M et al. Phase I trial of “bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer. Mol Ther 2012; 20: 679–686.
Strumberg D, Schultheis B, Traugott U, Vank C, Santel A, Keil O et al. Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int J Clin Pharmacol Ther 2012; 50: 76–78.
Rao DD, Maples PB, Senzer N, Kumar P, Wang Z, Pappen BO et al. Enhanced target gene knockdown by a bifunctional shRNA: a novel approach of RNA interference. Cancer Gene Ther 2010; 17: 780–791.
Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T . Identification of novel genes coding for small expressed RNAs. Science 2001; 294: 853–858.
Liu S, Ballian N, Belaguli NS, Patel S, Li M, Templeton NS et al. PDX-1 acts as a potential molecular target for treatment of human pancreatic cancer. Pancreas 2008; 37: 210–220.
Jay CM, Ruoff C, Kumar P, Maass H, Spanhel B, Miller M et al. Assessment of intravenous pbi-shRNA PDX1 nanoparticle (OFHIRNA-PDX1) in yucatan swine. Cancer Gene Ther 2013; 20: 683–689.
Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR et al. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 2006; 441: 537–541.
Giering JC, Grimm D, Storm TA, Kay MA . Expression of shRNA from a tissue-specific pol II promoter is an effective and safe RNAi therapeutic. Mol Ther 2008; 16: 1630–1636.
Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M et al. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol 2003; 21: 635–637.
Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 2013; 369: 819–829.
Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov 2013; 3: 406–417.
Kaiser PK, Symons RC, Shah SM, Quinlan EJ, Tabandeh H, Do DV et al. RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol 2010; 150: 33–39 e2.
Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Klamerus KJ, Chi-Burris K et al. Evaluation of the siRNA PF-04523655 versus ranibizumab for the treatment of neovascular age-related macular degeneration (MONET Study). Ophthalmology 2012; 119: 1867–1873.
Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ et al. Phase 1 dose-escalation study of a siRNA targeting the RTP801 gene in age-related macular degeneration patients. Eye 2012; 26: 1099–1105.
DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E et al. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci USA 2010; 107: 8800–8805.
DeVincenzo J, Cehelsky JE, Alvarez R, Elbashir S, Harborth J, Toudjarska I et al. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV). Antiviral Res 2008; 77: 225–231.
Zamora MR, Budev M, Rolfe M, Gottlieb J, Humar A, Devincenzo J et al. RNA interference therapy in lung transplant patients infected with respiratory syncytial virus. Am J Respir Crit Care Med 2011; 183: 531–538.
Gish RG, Satishchandran C, Young M, Pachuk C . RNA interference and its potential applications to chronic HBV treatment: results of a Phase I safety and tolerability study. Antivir Ther 2011; 16: 547–554.
Wyszko E, Rolle K, Nowak S, Zukiel R, Nowak M, Piestrzeniewicz R et al. A multivariate analysis of patients with brain tumors treated with ATN-RNA. Acta Pol Pharm 2008; 65: 677–684.
Acknowledgements
This research was supported by NCI Grant R01 CA95731, The Moss Foundation, Vivian Smith Foundation, MD Anderson Foundation and the H H Lee Research Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D Rao, CM Jay, P Kumar, N Senzer and N Templeton are employed by Gradalis. N Senzer, FC Brunicardi, D Rao and J Nemunaitis are shareholders in Gradalis.
Rights and permissions
About this article
Cite this article
Wu, J., Liu, S., Yu, J. et al. Vertically integrated translational studies of PDX1 as a therapeutic target for pancreatic cancer via a novel bifunctional RNAi platform. Cancer Gene Ther 21, 48–53 (2014). https://doi.org/10.1038/cgt.2013.84
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2013.84
Keywords
This article is cited by
-
Biological processes and signal transduction pathways regulated by the protein methyltransferase SETD7 and their significance in cancer
Signal Transduction and Targeted Therapy (2018)
-
Preclinical Justification of pbi-shRNA EWS/FLI1 Lipoplex (LPX) Treatment for Ewing's Sarcoma
Molecular Therapy (2016)