Abstract
Transforming growth factor-β (TGFβ) modulates the expression of multiple apoptotic target genes; however, a common and central signaling pathway, acting downstream of TGFβ and leading to cell death, has yet to be uncovered. Here, we show that TGFβ-induced apoptosis in cancer cells requires the transcription factor E2F1 (E2 promoter-binding factor 1). Using the E2F1 knockout mouse model, we also found E2F1 to be required for TGFβ-mediated apoptosis in normal cells. Moreover, we found TGFβ to increase E2F1 protein stability, acting at the post-translational level. We further investigated the molecular mechanisms by which E2F1 contributes to TGFβ-mediated apoptosis and found that TGFβ treatment led to the formation of a transcriptionally active E2F1–pRb–P/CAF complex on multiple TGFβ pro-apoptotic target gene promoters, thereby activating their transcription. Together, our findings define a novel process of gene activation by the TGFβ-E2F1 signaling axis and highlight E2F1 as a central mediator of the TGFβ apoptotic program.
Similar content being viewed by others
Main
Transforming growth factor-β (TGFβ) and its related family members are involved in the regulation of a wide range of fundamental cellular processes, including the regulation of growth, differentiation, and apoptosis.1 TGFβ, the prototype of the family, is a vital factor in the maintenance of homeostasis between cell growth and apoptosis. TGFβ exerts its tumor-suppressive effects by inhibiting cell-cycle progression, inducing apoptosis, and preventing immortalization through inhibition of telomerase activity. Loss or mutation of TGFβ signaling components is frequently observed in human cancer and further define a tumor-suppressive role for this growth factor.2
TGFβ ligands signal through serine/threonine kinase receptors that, once activated by ligand binding, recruit and phosphorylate the canonical downstream mediators, Smad2 and Smad3. Once phosphorylated, Smad2 and Smad3 interact with Smad4 to then translocate to the nucleus where the Smad complex associates with diverse DNA-binding factors to regulate expression of target genes in a cell- and tissue-specific manner. These partner proteins, which act as co-activators or co-repressors, are differentially expressed in different cell types and are thus thought to provide a basis for tissue and cell type-specific functions for TGFβ ligands.3
TGFβ induces a number of apoptotic responses and its ability to do so varies greatly depending on the cell type.4 Understanding the basis of this variability requires elucidating the molecular mechanisms involved in regulating TGFβ-mediated apoptosis. TGFβ signaling activates caspases in various epithelial cell types5, 6 and transcriptionally induces DAPK (death-associated protein kinase) in hepatoma cells.7 TGFβ also induces apoptosis by antagonizing PI3K (phosphatidylinositol 3-kinase)/Akt signaling activity through expression of the lipid phosphatase SHIP (SH2-domain-containing inositol-5-phosphatase) in hematopoietic cells.8 Transcriptional up-regulation of pro-apoptotic proteins such as Bax (Bcl-2-associated X protein) and down-regulation of pro-survival Bcl-2 (B-cell lymphoma 2) family members have also been implicated in TGFβ-mediated programmed cell death.9, 10 However, these mechanisms are context and tissue-specific; a central mechanism acting downstream of TGFβ to induce apoptosis has not yet been described.
We previously demonstrated that the TGFβ inhibitory effect on telomerase activity and cell immortalization is dependent on both Smad3 and the transcription factor E2F1 (E2 promoter-binding factor 1), highlighting E2F1 as an important mediator of TGFβ tumor-suppressive effects.11 The E2F family of transcription factors is a group of DNA-binding proteins that are central regulators of cell-cycle progression. The transcriptional activity of E2F1–5 is regulated primarily via their association with members of the retinoblastoma family of pocket proteins, which include pRb (retinoblastoma tumor-suppressor protein)/p105, p107, and p130.12 E2F1, the founding member and best-characterized of the family, has a unique role compared with other E2Fs, showing characteristics of being both an oncogene and a tumor suppressor, as it is able to induce both cell-cycle progression and apoptosis. Though an increase in E2F1 activity has been reported in several types of tumors13, 14 supporting an oncogenic role for E2F1, transgenic mice overexpressing E2F1 display aberrant cell apoptosis.15 Furthermore, E2F1 knockout mice develop highly malignant tumors and show defects in thymocyte apoptosis, highlighting E2F1 as a potent tumor suppressor.16 The nature of this dichotomy is proposed to be based on the degree to which E2F1 is expressed in the context of the cell cycle and/or following DNA damage, and the notion that different threshold levels of E2F1 are required for differential transactivation of its target gene promoters, which may favor either survival or apoptosis.17 Interestingly, E2F1 mutants that are unable to promote cell-cycle progression retain their ability to induce programmed cell death, indicating that induction of the cell cycle and apoptosis are separable functions of E2F1.18 Given our previous findings that E2F1 is required for TGFβ-mediated inhibition of hTERT (human telomerase reverse transcriptase)11 and that TGFβ promotes increased E2F-DNA-binding activity in pre-apoptotic hepatoma cell nuclear extracts,19 we investigated whether E2F1 could also mediate another arm of the TGFβ tumor-suppressive response and regulate apoptosis.
We found TGFβ to regulate the transcription of a number of pro-apoptotic genes in an E2F1-dependent manner in cancer cell lines from various tissues. Using embryonic fibroblasts from the E2F1 knockout mouse model, we also found E2F1 to be required for TGFβ-mediated apoptosis in normal cells. Moreover, we found TGFβ to increase E2F1 protein stability, acting post-translationally. We further investigated the molecular mechanisms by which E2F1 contributes to TGFβ-mediated cell death and found that TGFβ could promote formation of a transcriptionally active E2F1–pRb–P/CAF (p300/CREB-binding protein-associated factor) complex onto the promoters of TGFβ-targeted apoptotic genes to activate their transcription. Together, our results underline E2F1 as a central mediator of the TGFβ pro-apoptotic response and highlight the E2F1–pRb–P/CAF signaling pathway as a critical regulator of TGFβ-mediated cell death.
Results
TGFβ-mediated apoptosis is dependent on E2F1
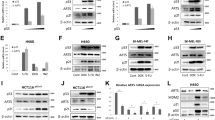
We first examined the pro-apoptotic effect of TGFβ in various model systems, including two human hepatoma cell lines (HuH7 and HepG2), a human melanoma cell line (WM278), and a human keratinocyte cell line (HaCaT). Cells were stimulated or not with TGFβ as indicated and apoptosis was assessed using MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) cell viability assay as well as calcein-AM (calcein-acetoxymethyl ester) assay, a more sensitive assay for early apoptosis detection.20 All cell lines tested were strongly growth inhibited by TGFβ treatment in a time-dependent manner (Figures 1a and b). To address the contribution of E2F1 in mediating this TGFβ response, we used RNA interference to reduce the expression of endogenous E2F1. Interestingly, we found that the effect of TGFβ on cell viability (Figure 1c) and early apoptosis (Figure 1d) in all the cell lines tested was almost completely prevented when E2F1 expression was silenced, indicating that E2F1 is required for mediating the TGFβ pro-apoptotic response in multiple cell lines of various origins.
TGFβ-mediated apoptosis is dependent on E2F1. (a and b) The specified cell lines were untreated or treated with TGFβ (100 pM) for the indicated times and assessed for cell viability by (a) MTT and (b) calcein-AM assays. Data are represented as mean±S.D. (c, d) Cells were transiently transfected with two different siRNAs against E2F1 or a control non-silencing siRNA and assessed by (c) MTT and (d) calcein-AM assays. (e) The efficiency of E2F1 knockdown by siRNA was verified by immunoblotting with an E2F1 specific antibody. (f and g) Activation of the apoptotic program by TGFβ was assessed by AnnexinV staining followed by (f) FACS or (g) fluorescence microscopy, in HuH7 cells transiently transfected with a control, non-targeting siRNA, or E2F1 siRNA. In (f), values represent the percentage of early and late apoptotic cells and represent the mean±S.D. (h) Expression of endogenous E2F1 in these cells was assessed by immunofluorescence
To further investigate the role of E2F1 in TGFβ-mediated apoptosis, we performed fluorescence-activated cell sorting (FACS) following AnnexinV and propidium iodide staining. Although TGFβ treatment markedly increased the number of apoptotic cells in control siRNA-transfected HuH7 cells (Figure 1f, left panels), E2F1 knockdown completely abolished this effect (Figure 1f, right panels), consistent with cell viability and calcein-AM results. Fluorescence imaging following AnnexinV staining further confirmed these findings (Figure 1g). Taken together, these results indicate that TGFβ has a strong pro-apoptotic function in various cell lines and that these effects require the transcription factor E2F1.
E2F1 is required for TGFβ-mediated regulation of pro-apoptotic target genes
TGFβ signaling activates multiple pro-apoptotic genes and pathways in a cell- and tissue-specific manner.4 Independently of TGFβ, the E2F pathway is also involved in multiple distinct apoptotic mechanisms. In varying cell types and tissues, E2F1 alone has been shown to activate numerous pro-apoptotic genes, including Apaf1 (apoptotic protease activating factor 1), p14ARF, p73, Caspase 3, Caspase 7, Caspase 8, Chk2 (checkpoint kinase 2), Ask-1 (apoptosis signal-regulating kinase 1), and Smac/DIABLO (second mitochondrial-derived activator of caspase/direct IAP-binding protein with low pI).21, 22, 23, 24, 25, 26, 27
To assess whether TGFβ and E2F1 share any common downstream apoptotic targets, we examined the regulation of representative E2F1-responsive pro-apoptotic genes in TGFβ-treated human hepatoma HuH7 cells, which express both functional p53 and pRb. As shown in Figure 2a, TGFβ potently induced mRNA expression of Apaf1, Caspase 3, Caspase 7, p73, and Smac/DIABLO, suggesting that TGFβ induces apoptosis in HuH7 cells by the intrinsic mitochondrial pathway. Importantly, this analysis also revealed Smac/DIABLO as a novel TGFβ target. Loss of E2F1 expression markedly impaired the TGFβ-mediated induction of each of these target genes (Figure 2b), indicating that E2F1 is required for TGFβ-mediated regulation of its pro-apoptotic downstream target genes. Moreover, these data provide a novel pathway by which TGFβ regulates these genes and reveals E2F1 as a widespread co-transducer of TGFβ-induced activation of the intrinsic mitochondrial pathway.
E2F1 is required for TGFβ-mediated regulation of proapoptotic genes. (a) HuH7 cells were stimulated with TGFβ (100 pM) and mRNA levels for the indicated genes were measured by real-time qPCR. Results are normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and shown relative to levels observed in untreated cells (set to 1). Data are represented as mean±S.D. (b) HuH7 cells were transiently transfected with siRNA against E2F1 or a control non-silencing siRNA and stimulated with TGFβ (100 pM) for 24 h. The mRNA levels for the indicated genes were measured as in (a). (c) HuH7 cells were pre-treated for 30 min with cycloheximide (10 mu;M) or vehicle and then stimulated with TGFβ (100 pM) for the indicated times. The mRNA levels for the indicated genes were analyzed by RT-PCR and the amplified products were analyzed by DNA gel electrophoresis
To then examine whether these pro-apoptotic genes are direct targets of TGFβ, cells were treated or not with the translational inhibitor cycloheximide (CHX) and stimulated with TGFβ as indicated. Interestingly, CHX treatment of the cells completely impaired the induction of these genes by TGFβ (Figure 2c). As a control, the mRNA expression levels of a direct TGFβ target gene, Smad7, were also examined and, as expected, were not affected by CHX treatment. These results indicate that TGFβ regulation of expression of its downstream pro-apoptotic target genes is indirect and requires the induction of a TGFβ-responsive transcriptional activator.
TGFβ rapidly and transiently induces E2F1 protein expression levels
Having shown that TGFβ indirectly induces the expression of these pro-apoptotic target genes and that E2F1 is required for this process, we next sought to determine whether E2F1 expression itself was regulated by TGFβ. TGFβ treatment induced a time-dependent decrease in E2F1 mRNA levels in HaCaT cells (Figure 3a), in agreement with previous reports.28, 29 Surprisingly, however, we found TGFβ to rapidly and transiently induce E2F1 protein expression levels in these cells (Figure 3b). We then examined the TGFβ effect on E2F1 protein expression levels in human epithelial cancer cell lines originating from different tissues (melanoma, hepatocarcinoma, and colon carcinoma) and, as shown in Figure 3c, E2F1 protein levels were strongly induced by TGFβ in all the cell lines tested. This effect was transient, however, as longer exposure to TGFβ resulted in a return to basal E2F1 protein levels. Interestingly, in all cases the increase in E2F1 expression in response to TGFβ was very rapid, suggesting that TGFβ induces post-translational protein stabilization of E2F1. To address this, we performed a CHX chase in HaCaT cells treated or not with TGFβ (Figure 3d). In the presence of CHX, untreated cells showed progressive diminished levels of E2F1 over time. Conversely, TGFβ treatment maintained E2F1 levels throughout the chase, indicating that TGFβ indeed prolongs E2F1 half-life, by stabilizing E2F1 protein levels post-translationally.
TGFβ rapidly and transiently induces E2F1 protein expression levels. HaCaT cells were stimulated with TGFβ (100 pM) for the indicated times and subjected to (a) RT-PCR followed by DNA gel electrophoresis and (b) western blotting to measure E2F1 RNA and protein levels, respectively. (c) Western blot analysis of total E2F1 protein levels in TGFβ-treated cells of various origins, as indicated. (d) Cycloheximide (CHX) chase analysis in HaCaT cells to address the potential contribution of TGFβ in E2F1 post-translational stabilization. Cells were incubated with CHX (50 μg/mL) and treated or not with TGFβ (100 pM) for the indicated times. Total cell lysates were analyzed for E2F1 protein levels by western blotting
TGFβ pro-apoptotic effects are impaired in E2F1-null embryonic fibroblasts
Having shown that TGFβ-induced apoptosis in various epithelial cancer cell lines requires E2F1, we next examined the contribution of E2F1 downstream of TGFβ-mediated cell death in normal cells. For this, we used mouse embryonic fibroblasts (MEFs) isolated from wild-type and E2F1-deficient mice. Importantly, both wild-type (E2F1+/+) and E2F1-null (E2F1−/−) MEFs respond equally to TGFβ stimulation, as assessed by the induction of Smad phosphorylation (Figure 4a). The pro-apoptotic effect of TGFβ, however, greatly differed in these two cell types. Athough cell viability of the wild-type E2F1+/+ MEFs was potently decreased in response to TGFβ, this effect was severely impaired in the E2F1−/− MEFs (Figure 4b). Correspondingly, TGFβ-induced expression of Caspase 7 and Smac/DIABLO was significantly reduced in the E2F1−/− MEFs (Figure 4c). Together, these findings highlight a critical role for E2F1 downstream of TGFβ in the mediation of apoptosis in a normal cell setting in addition to multiple cell lines of various cancer origins.
TGFβ pro-apoptotic effects are impaired in E2F1-null embryonic fibroblasts. (a) Wild-type (WT) and E2F1−/− mouse embryonic fibroblasts (MEFs) were untreated or treated with TGFβ (100 pM) for the indicated times and phospho-Smad3 levels of total cell lysates were analyzed by western blotting. (b) WT and E2F1−/− MEFs were stimulated or not with TGFβ (100 pM) for 24 h and cell viability assessed by calcein-AM assay. (c) Caspase 7 and Smac/DIABLO mRNA levels in TGFβ-treated WT and E2F1−/− MEFs were measured by real-time qPCR analysis. Results are normalized to GAPDH and shown relative to levels observed in untreated cells (set to 1). Data are represented as mean±S.D. (*P<0.05)
E2F1 DNA-binding, transactivation, and pRb-interaction are required for TGF β -mediated apoptosis
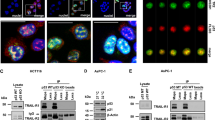
To further understand the molecular mechanisms underlying the role of E2F1 in the induction of programmed cell death by TGFβ, we next addressed the contribution of E2F1’s principal regulator, pRb. For this, we used dominant-negative E2F1 mutant forms to alter E2F1 function and/or binding to pRb. Importantly, the DNA-binding-deficient mutant, E2F1 (E132), and the transactivation-defective mutant, E2F1 (1–374), are both reportedly unable to activate transcription, whereas the E2F1 Y411C mutant, which has lost its ability to interact with pRb, retains similar transcriptional-activating potential as its wild-type E2F1.30 Interestingly, transient overexpression of each of these mutants drastically impeded the effect of TGFβ on cell viability in HuH7 cells (Figure 5a). The antagonistic effects of these E2F1 mutants were further established at the transcriptional level, as their overexpression significantly reduced TGFβ-induced Caspase 7 and Smac/DIABLO mRNA levels (Figure 5b). These results indicate that TGFβ requires not only proper E2F1 function (DNA binding and transactivation), but the ability of E2F1 to interact with pRb in order to successfully induce apoptosis. To further address this, we examined whether TGFβ could induce association between endogenous E2F1 and pRb using co-immunoprecipitation studies. As shown in Figure 5c, TGFβ treatment indeed promotes the association between E2F1 and pRb. Altogether, these results indicate that pRb-E2F binding is required for TGFβ to induce apoptosis and that this association is induced by TGFβ itself, strongly supporting the fact that the pRb-E2F1 protein complex has a role downstream of TGFβ-mediated cell signaling, leading to apoptosis.
E2F1 DNA-binding, transactivation, and pRb-interaction are required for TGFβ-mediated apoptosis. HuH7 cells transiently transfected with empty vector or mutant E2F1 expression constructs as indicated were untreated or treated with 100 pM TGFβ for 24 h. (a) Cell viability was assessed by calcein-AM assay, with bars representing means±S.D. (b) Caspase 7 and Smac/DIABLO mRNA levels were measured by real-time qPCR analysis. Results are normalized to GAPDH and show the mean±S.D., expressed as relative to levels observed in untreated cells (set to 1). (c) HuH7 cells untreated or treated with TGFβ (100 pM) were subjected to immunoprecipitation (IP) with the specified antibodies followed by western blotting (WB) to assess levels of associated E2F1 and pRb
TGFβ induces formation of a transcriptionally active complex between pRb/E2F1 and the acetyltransferase P/CAF onto pro-apoptotic gene promoters
Given the classical model of E2F regulation, which implies that E2F1 must be in its unbound form in order to activate transcription, this raised the question as to how E2F1 activates these pro-apoptotic genes in response to TGFβ while remaining in its seemingly transcriptionally repressive pRb-E2F complex. Thus, we assessed whether TGFβ could in fact recruit positive regulators of transcription to the pRb-E2F1 complex. As TGFβ may activate gene transcription through histone acetyltransferases, including p300/CBP (cAMP-response element-binding protein (CREB)-binding protein) and P/CAF (p300/CBP-associated factor),31 we screened for the presence of these histone acetyltransferases in E2F1 and pRb immunoprecipitates in untreated versus TGFβ-treated cells. Interestingly, as shown in Figure 6a, we found that TGFβ strongly promotes the association of both E2F1 and pRb to the acetyltransferase P/CAF. Moreover, these complexes appear to be P/CAF specific as we could not detect any association between pRb-E2F1 and p300/CBP.
TGFβ induces formation of a transcriptionally active complex between pRb/E2F1 and the acetyltransferase P/CAF, onto pro-apoptotic gene promoters. (a) Untreated and TGFβ-treated HuH7 cells were subjected to immunoprecipitation (IP) with the specified antibodies followed by western blotting (WB) to assess levels of P/CAF or CBP/p300 and associated E2F1 and pRb. (b and c) HuH7 cells were transiently transfected with siRNA against P/CAF or a control non-silencing siRNA and treated with TGFβ (100 pM) for 24 h. Cell viability was assessed by (b) calcein-AM assay, and Caspase 7 and Smac/DIABLO mRNA levels were measured by (c) real-time qPCR analysis. Results are normalized to GAPDH and shown relative to levels observed in untreated cells (set to 1). Data are represented as mean±S.D. (d) The efficiency of P/CAF knockdown by siRNA was verified by real-time qPCR. (e) HuH7 cells were transiently transfected with a control siRNA or siRNA again P/CAF (left panel) or E2F1 (right panel) and treated with TGFβ (100 pM) as indicated. Activation of Caspase 3/7 was measured by Caspase-Glo 3/7 assay (Promega). Data are represented as mean±S.D. (f) HuH7 cells were untreated or treated with TGFβ (100 pM) for the indicated times, and the binding of E2F1, pRb, and P/CAF to the p73, Apaf1, Smac/DIABLO, and Caspase 7 gene promoters was determined by chromatin immunoprecipitation (ChIP)
We then addressed whether P/CAF is required for the activation of E2F1-responsive pro-apoptotic genes and induction of apoptosis in response to TGFβ. Loss of P/CAF expression by RNA interference dramatically reduced the TGFβ pro-apoptotic effect in these cells (Figure 6b). Moreover, the TGFβ-induced expression levels of Caspase 7 and Smac/DIABLO were notably reduced when P/CAF expression was silenced by siRNA (Figure 6c). As caspases require post-translational activation to become catalytically active and mediate cell death,32 we investigated whether the loss of TGFβ-induced caspase expression due to P/CAF knockdown was followed by a decrease in caspase activity. As shown in Figure 6e (left panel), blocking P/CAF expression severely impaired TGFβ-mediated Caspase 3/7 activation. This effect was similar to what was observed when E2F1 expression was silenced (Figure 6e, right panel). By 48 h, loss of either P/CAF or E2F1 expression nearly completely abolished TGFβ-induced caspase activation. Collectively, these findings support a critical role for P/CAF downstream of TGFβ in the E2F1-dependent activation of pro-apoptotic genes and the mediation of programmed cell death.
To then assess the functional relevance of the TGFβ-induced pRb-E2F1-P/CAF complex in regulating TGFβ transcriptional responses, we performed chromatin immunoprecipitation assays to determine whether this complex is recruited to the pro-apoptotic target gene promoters in response to TGFβ. We examined the promoters of the TGFβ- and E2F1-responsive pro-apoptotic genes identified above. Interestingly, as shown in Figure 6f, TGFβ treatment markedly induced recruitment of all three partners (E2F1, pRb, and P/CAF) to the p73, Apaf1, Caspase 7, and Smac/DIABLO gene promoters, concurring with the TGFβ-mediated increase in the mRNA levels of these pro-apoptotic genes and activation of the apoptotic program. These results highlight the E2F1–pRb–P/CAF pathway as a major signaling axis leading to apoptosis downstream of TGFβ in normal and cancer cells.
Discussion
Although various apoptotic mediators and signaling pathways have been implicated in TGFβ-mediated apoptosis, most of these regulatory mechanisms appear to be cell type-dependent or tissue-specific.4 This study defines a novel process of gene activation by the TGFβ–E2F1 signaling axis, and highlights the pRb–E2F1–P/CAF pathway as a wide-ranging and critical mediator of the TGFβ apoptotic program in multiple target tissues.
We identified a number of key pro-apoptotic TGFβ target genes that trigger the intrinsic apoptosis pathway through the induction of E2F1. Although these genes are functionally interrelated, our results imply that TGFβ regulates the intrinsic apoptosis pathway at multiple levels, consistent with the strong pro-apoptotic effect of this growth factor in its target tissues. However, we do not exclude the possibility that induction of other targets (or pathways) might also contribute to E2F1-dependent TGFβ-mediated cell death. Importantly, these results are corroborated using the E2F1 knockout mouse model, demonstrating that the TGFβ–E2F1 signaling pathway mediates TGFβ-induced cell death not only in a diseased state but in a normal cell setting as well.
Although it is well-established that E2F1 activity is intimately controlled through association with pRb, the precise mechanisms of this regulation are somewhat contradictory. The prevailing view holds that the pRb–E2F1 complex acts as a repressor of E2F target genes.12 Accordingly, disruption of this pRb–E2F1 complex is required to release free E2F1 in order to induce transcription of its target genes. Paradoxically, pRb–E2F1 complexes were recently shown to transcriptionally activate pro-apoptotic genes in response to DNA damage through recruitment of a histone acetyltransferase to the pRb–E2F1 complex.33 Interestingly, our results also challenge this dogma, and support a non-classic transcriptionally active pRb–E2F1 regulatory complex, as we show here that the pRb–E2F1 complex can also recruit an actyltransferase (P/CAF) to activate transcription of pro-apototic genes in response to TGFβ. Indeed, analysis with dominant-negative E2F1 mutants revealed that, in fact, pRb binding to E2F1 is required for TGFβ-mediated apoptosis.
Our results also indicate that TGFβ rapidly increases E2F1 protein levels, acting at the post-translational level. Interestingly, several lines of evidence have demonstrated that the E2Fs are often regulated by post-translational modifications such as phosphorylation,34 acetylation,35 and by the ubiquitin–proteasome pathways.36 Binding of pRb to E2F1 protects E2F1 from ubiquitination and proteolytic degradation,37 thereby increasing its stability. As TGFβ maintains pRb in a hypophosphorylated form, causing E2F1 to remain bound to pRb and suppressing activation of E2F1-responsive cell-cycle regulatory genes,38 it is likely that the TGFβ effect on E2F1 protein levels is mediated through induction of pRb-E2F1 association, revealing a new level of E2F1 regulation.
Moreover, the association of P/CAF to E2F1 may also contribute to the increased stability of E2F1 protein levels in response to TGFβ, as P/CAF also binds and acetylates E2F1, prolonging its half-life. In fact, E2F1 acetylation by P/CAF has three functional effects on E2F1 activity: increased protein-half life, DNA-binding ability, and activation potential.35 Thus, P/CAF binding to E2F1 in response to TGFβ may in fact have multiple functional consequences, affecting not only E2F1 stability but its transcriptional-activating capability as well.
Additional post-translational modifications of E2F1 and/or pRb may also contribute to the formation of the pro-apoptotic complex. Notably, pRb holds a second alternate E2F1-specific binding site that does not interfere with E2F1’s transactivation domain.39 It is interesting to consider, then, whether TGFβ could somehow induce pRb and E2F1 to assume this alternate conformation. If so, this conformation should also allow for recruitment of P/CAF, which we have demonstrated here to be required for TGFβ to activate E2F1-dependent pro-apoptotic target genes. The coordinated recruitment of E2F1, pRb, and P/CAF to these pro-apoptotic gene promoters suggests the potential formation of a transcriptionally active pRb–E2F1 complex, which mediates the regulation of TGFβ pro-apoptotic targets. Taken together, these results strongly support a pro-apoptotic role for the E2F1 pathway downstream of TGFβ and provide a potential mechanism for the activation of E2F1-responsive pro-apoptotic genes in response to TGFβ.
It is interesting to consider that TGFβ tumor-suppressive effects might utilize the functional interplay among the E2F family members, which affects E2F activity. It is well-established that TGFβ prevents cell-cycle progression, causing G1 arrest, by up-regulating expression of Cdk (cyclin-dependent kinase) inhibitors and by inhibiting both cdc25a (cell division cycle 25 homolog A)40 and c-myc41 by means of Smad–E2F4/5–pocket protein repressor complexes. The rapid surge in E2F1 that we observe in response to TGFβ may thus effectively initiate the TGFβ apoptotic program, without affecting cell cycle, as TGFβ maintains transcriptional repression of factors required for S-phase entry through the other E2F family members. Moreover, E2F4, in complex with pRb or p107, is capable of binding to E2F-binding sites on the E2F1 promoter, leading to its repression after 4 h of TGFβ treatment.28 Thus, it is conceivable that TGFβ treatment leads to increased levels of E2F1, triggering the activation of pro-apoptotic genes. Subsequently, in addition to directly inhibiting cell-cycle regulatory genes, E2F4 may repress E2F1 levels following longer stimulation with TGFβ, further preventing cell-cycle progression.
The present work delineates a novel process of gene activation by the TGFβ–E2F1 signaling axis and supports a role for the E2F family as potent co-transducers of TGFβ signals. Combined with previous studies from our lab and others, these findings highlight the crucial role for the E2F family in regulating TGFβ tumor-suppressive effects and we propose the following model of E2F tumor-suppressive action downstream of TGFβ (Figure 7):
-
1)
TGFβ induces E2F4/5 recruitment into classical repressive pRb–E2F–HDAC (histone deacetylase) complexes, which target key cell-cycle regulators, such as cdc25a40 and c-myc,41, 42 preventing cell-cycle entry.
-
2)
TGFβ also induces E2F1 recruitment into repressive E2F–HDAC complexes, inhibiting hTERT expression and suppressing immortalization, as we have previously demonstrated.11
-
3)
The current study demonstrates that TGFβ can also recruit E2F1 into transcriptionally active pRb–E2F1–P/CAF complexes, increasing the expression of multiple pro-apoptotic target genes and inducing programmed cell death.
It is interesting to note that the E2F family acts via distinct pathways to regulate specific genes, yet all toward a global action of tumor suppression. We can thus consider the E2F family as ‘super-mediators’ of TGFβ tumor-suppressive effects. A better understanding of the mechanisms by which both TGFβ and E2F1 exert their tumor-suppressive roles may prove useful for the development of novel therapeutic strategies aimed at restoring the apoptotic or tumor-suppressive response of the E2Fs in human cancer.
Materials and Methods
Cell culture and transfections
HaCaT, HuH7, HepG2, Moser, and SKCO cell lines, as well as MEFs were cultured in DMEM (HyClone, Logan, UT, USA) and WM278 cells in RPMI-1640 (HyClone). Medium for all cells was supplemented with 10% fetal bovine serum (FBS) (HyClone) and 2 mM L-glutamine (GIBCO, Grand Island, NY, USA), and cells were grown at 37 °C in 5% CO2 conditions. Before treatment, cells were serum-starved for 24 h and all stimulations were done in serum-free medium containing 100 pM TGFβ1 (PeproTech, Rocky Hill, NJ, USA). Cells were transiently transfected with different siRNAs against E2F1 (Ambion, Foster City, CA, USA) or P/CAF (Sigma-Aldrich, St. Louis, MO, USA), or with wild-type and mutant E2F1 expression vectors using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions.
Viability assays
Cells were seeded in triplicate in 96-well plates, at 10 000 cells/100 μl in medium supplemented with 2% FBS, and in the presence or absence of 100 pM TGFβ. Mitochondrial viability was determined by MTT colorimetric assay. Briefly, following 24–72 h of TGFβ treatment, cells were incubated with 1 mg/ml MTT solution (Sigma-Aldrich) in the culture media for 2 h. Formazan crystals were solubilized overnight in 50% dimethyl formamide, 20% SDS, pH 4.7, and the absorbance of each well was measured at 570 nm using a microplate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA). Alternatively, cell viability was determined by the fluorescent calcein-AM method. Briefly, following 4–24 h of TGFβ treatment, original culture medium was replaced with serum-free medium containing 2 μg/ml calcein-AM (BD Biosciences, San Diego, CA, USA) for 60 min at 37 °C. Cells were then washed twice with PBS and the fluorescence of each well was monitored from the bottom of the wells at excitation and emission wavelengths of 485 and 520 nm, respectively, using a FLUOstar Optima microplate reader (BMG Labtech, Ortenberg, Germany).
RNA isolation and real-time quantitative PCR
Total RNA was isolated from cell lines using TRIzol reagent (Invitrogen) and reverse transcribed using random hexamers and M-MLV Reverse Transcriptase (Invitrogen), as per the manufacturer’s instructions. Subsequently, real-time qPCR was carried out using SsoFast EvaGreen Supermix (Bio-Rad, Hercules, CA, USA) in a RotorGene 6000 PCR detection system (Corbett Life Science, Montreal Biotech Inc., Kirkland, QC, Canada). The conditions for qPCR were as follows: 95 °C for 30 s, 40 cycles of 95 °C for 5 s, and 60 °C for 20 s. Primer sequences are listed in Table 1. Where indicated, some cDNAs were amplified for 30 cycles instead and amplified products were analyzed by DNA gel electrophoresis.
Immunoblotting and immunoprecipitation
Cells were lysed in cold RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM EDTA), containing 1 mM sodium orthovanadate, 1 mM phenylmethylsulphonyl fluoride, 5 μg/ml aprotinin, 2 μg/ml leupeptin, and 1 μg/ml pepstatin. Lysates were separated by SDS-PAGE, transferred to nitrocellulose, and incubated with the specified antibodies overnight at 4 °C: anti-E2F1 (KH95, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-β-tubulin (Sigma-Aldrich), and anti-phospho-Smad3 (BioSource, Camarillo, CA, USA). Following primary antibody incubation, membranes were washed twice in TBST (50 mM Tris-HCl at pH 7.6, 200 mM NaCl, 0.05% Tween20) and incubated with secondary antibody coupled to horseradish peroxidase (Sigma-Aldrich) at 1 : 10 000 dilution for 1 h at room temperature. Membranes were then washed in TBST four times for 15 min. Immunoreactivity was revealed by chemiluminescence and detected using an Alpha Innotech Fluorochem Imaging system (Packard Canberra, Montreal, QC, Canada). Immunoprecipitations were performed overnight at 4 °C using antibodies against E2F1 (C-20, Santa Cruz Biotechnology), pRb (Cell Signaling, Danvers, MA, USA), P/CAF (Abcam, Cambridge, MA, USA), and CBP/p300 (Santa Cruz Biotechnology). Protein A-sepharose (Amersham Biosciences, Uppsala, Sweden) was added for 2 h at 4 °C, and beads were then washed four times with cold lysis buffer. The immunoprecipitates were eluted with 2 × SDS Laemmli sample buffer, boiled for 5 min, and subjected to immunoblotting.
Annexin-V apoptotic assays
Apoptotic cells were analyzed using an Annexin V apoptosis detection kit (Santa Cruz Biotechnology). Following TGFβ treatment, cells were collected by trypsinization, pelleted by centrifugation, washed with PBS, and each sample was incubated with 0.5 μg Annexin V-FITC and 10 μl propidium iodide (50 μg/ml) in the supplied incubation buffer for 15 min. Cells were then analyzed using FACS in an Accuri C6 flow cytometer (BD Biosciences). For fluorescence microscopy, cells were plated on glass coverslips at 80% confluence. Following TGFβ treatment, cells were washed with PBS and subjected to Annexin V-FITC staining for 15 min as described above. Stained coverslips were mounted onto slides with SlowFade Gold Antifade with DAPI (Invitrogen), and immediately examined.
Immunofluorescence
Cells plated on glass coverslips were fixed with 4% paraformaldehyde, permeabilized in PBS containing 0.1% Triton X-100 for 3 min, washed with PBS, and blocked with 2% bovine serum albumin (BSA) for 30 min. Cells were then incubated with anti-E2F1 antibody (Santa Cruz Biotechnology) for 1 h, washed with PBS, and incubated with AlexaFluor568 goat anti-mouse IgG secondary antibody (Invitrogen) for 1 h. After a final wash, stained coverslips were mounted with SlowFade Gold Antifade with DAPI (Invitrogen) and examined using a Zeiss LSM-510 Meta Axiovert confocal microscope (Carl Zeiss, Thornwood, NY, USA).
Caspase activity
Cells were plated in triplicate in 96-well dishes, at 10 000 cells/100 μl in medium supplemented with 2% FBS, and in the presence or absence of 100 pM TGFβ. Caspase 3/7 activity was measured using the Caspase-Glo 3/7 Assay (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Briefly, following TGFβ treatment, cells were incubated with Caspase-Glo reagent (Promega) for 1.5 h at room temperature, and the luminescence of each sample was measured using a luminometer (EG & G Berthold, Bad Wildbad, Germany).
CHX chase
Cells were seeded in 60-mm2 plates and grown to 85% confluence. Following overnight serum-starvation, the cells were incubated, in the presence or absence of 100 pM TGFβ, with 50 μg/ml CHX (Sigma-Aldrich) for the indicated times and analyzed by immunoblotting.
Chromatin immunoprecipitation
Protein complexes were cross-linked to DNA by adding formaldehyde directly to tissue culture medium to a final concentration of 1%. Crosslinking was allowed to proceed for 10 min at room temperature and was then stopped by the addition of glycine to a final concentration of 0.125 M. Cross-linked cells were harvested, washed with PBS, pelleted by centrifugation at 2000, r.p.m. for 5 min at 4 °C, and lysed in nuclear lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl (pH 8.1)), supplemented with 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 2 μg/ml pepstatin, for 10 min on ice. The resulting chromatin solution was sonicated for five pulses of 20 s to generate 300–2000 bp DNA fragments. After centrifugation at 14 000 r.p.m. for 10 min at 4 °C, the supernatant was immunocleared by incubation with protein A-sepharose beads for 2 h at 4 °C. Immunocleared chromatin was immunoprecipitated overnight with 5 μg of the indicated antibodies. Antibody–protein–DNA complexes were then isolated by immunoprecipitation with 40 μl protein A-sepharose beads (Amersham) for 2 h with rotation at 4 °C. Beads were washed consecutively for 10 min each with low-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 20 mM Tris-HCl, pH 8.1), high-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 500 mM NaCl, 20 mM Tris-HCl, pH 8.1), and LiCl wash buffer (0.25 M LiCl, 1% NP-40, 1% Na-deoxycholate, 1 mM EDTA, 10 mM Tris-HCl, pH 8.1), and twice in TE buffer. Complexes were then eluted twice in 150 μl of freshly made elution buffer (1% SDS, 0.1 M NaHCO3) by incubating at 65 °C for 10 min. To reverse cross-linking, 0.2 M NaCl and 1 μl of 10 mg/ml RNaseA was added to each sample, and they were incubated at 65 °C overnight. Following this, 5 mM EDTA and 2 μl of 10 mg/ml proteinase K was added, and samples were incubated for at 45 °C for 2 h. DNA was recovered using the QIAquick spin columns (Qiagen, MD, USA) as per the manufacturer’s protocol and PCR analysis was performed using primers specific for the indicated promoters, as listed in Table 2.
Statistical analysis
Results are expressed as mean±standard deviation of at least three independent experiments. Statistical differences were determined by two-tailed unpaired t-test. P<0.05 was considered statistically significant.
Abbreviations
- CHX:
-
cycloheximide
- E2F:
-
E2 promoter-binding factor
- MEF:
-
mouse embryonic fibroblast
- P/CAF:
-
p300/CREB-binding protein-associated factor
- PI3K:
-
Phosphatidylinositol 3-kinase
- pRb:
-
retinoblastoma tumor-suppressor protein
- Smac/DIABLO:
-
second mitochondrial-derived activator of caspase/direct IAP-binding protein with low pI
- TGFβ:
-
transforming growth factor-β
References
Massague J . The transforming growth factor-beta family. Annu Rev Cell Biol 1990; 6: 597–641.
Pardali K, Moustakas A . Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta 2007; 1775: 21–62.
Massague J, Wotton D . Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 2000; 19: 1745–1754.
Siegel PM, Massague J . Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 2003; 3: 807–821.
Ohgushi M, Kuroki S, Fukamachi H, O’Reilly LA, Kuida K, Strasser A et al. Transforming growth factor beta-dependent sequential activation of Smad Bim, and caspase-9 mediates physiological apoptosis in gastric epithelial cells. Mol Cell Biol 2005; 25: 10017–10028.
Kim SG, Kim SN, Jong HS, Kim NK, Hong SH, Kim SJ et al. Caspase-mediated Cdk2 activation is a critical step to execute transforming growth factor-beta1-induced apoptosis in human gastric cancer cells. Oncogene 2001; 20: 1254–1265.
Jang CW, Chen CH, Chen CC, Chen JY, Su YH, Chen RH . TGF-beta induces apoptosis through Smad-mediated expression of DAP-kinase. Nat Cell Biol 2002; 4: 51–58.
Valderrama-Carvajal H, Cocolakis E, Lacerte A, Lee EH, Krystal G, Ali S et al. Activin/TGF-beta induce apoptosis through Smad-dependent expression of the lipid phosphatase SHIP. Nat Cell Biol 2002; 4: 963–969.
Motyl T, Grzelkowska K, Zimowska W, Skierski J, Wareski P, Ploszaj T et al. Expression of bcl-2 and bax in TGF-beta 1-induced apoptosis of L1210 leukemic cells. Eur J Cell Biol 1998; 75: 367–374.
Ramjaun AR, Tomlinson S, Eddaoudi A, Downward J . Upregulation of two BH3-only proteins, Bmf and Bim, during TGF beta-induced apoptosis. Oncogene 2007; 26: 970–981.
Lacerte A, Korah J, Roy M, Yang XJ, Lemay S, Lebrun JJ . Transforming growth factor-beta inhibits telomerase through SMAD3 and E2F transcription factors. Cell Signal 2008; 20: 50–59.
Dyson N . The regulation of E2F by pRB-family proteins. Genes Dev 1998; 12: 2245–2262.
Suzuki T, Yasui W, Yokozaki H, Naka K, Ishikawa T, Tahara E . Expression of the E2F family in human gastrointestinal carcinomas. Int J Cancer 1999; 81: 535–538.
Eymin B, Gazzeri S, Brambilla C, Brambilla E . Distinct pattern of E2F1 expression in human lung tumours: E2F1 is upregulated in small cell lung carcinoma. Oncogene 2001; 20: 1678–1687.
Wang D, Russell JL, Johnson DG . E2F4 and E2F1 have similar proliferative properties but different apoptotic and oncogenic properties in vivo. Mol Cell Biol 2000; 20: 3417–3424.
Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ . Tumor induction and tissue atrophy in mice lacking E2F-1. Cell 1996; 85: 537–548.
Crosby ME, Almasan A . Opposing roles of E2Fs in cell proliferation and death. Cancer Biol Ther 2004; 3: 1208–1211.
Phillips AC, Bates S, Ryan KM, Helin K, Vousden KH . Induction of DNA synthesis and apoptosis are separable functions of E2F-1. Genes Dev 1997; 11: 1853–1863.
Fan G, Ma X, Kren BT, Steer CJ . Unbound E2F modulates TGF-beta1-induced apoptosis in HuH-7 cells. J Cell Sci 2002; 115 (Pt 15): 3181–3191.
Gatti R, Belletti S, Orlandini G, Bussolati O, Dall'Asta V, Gazzola GC . Comparison of annexin V and calcein-AM as early vital markers of apoptosis in adherent cells by confocal laser microscopy. J Histochem Cytochem 1998; 46: 895–900.
Furukawa Y, Nishimura N, Satoh M, Endo H, Iwase S, Yamada H et al. Apaf-1 is a mediator of E2F-1-induced apoptosis. J Biol Chem 2002; 277: 39760–39768.
Elliott MJ, Dong YB, Yang H, McMasters KM . E2F-1 up-regulates c-Myc and p14(ARF) and induces apoptosis in colon cancer cells. Clin Cancer Res 2001; 7: 3590–3597.
Irwin M, Marin MC, Phillips AC, Seelan RS, Smith DI, Liu W et al. Role for the p53 homologue p73 in E2F-1-induced apoptosis. Nature 2000; 407: 645–648.
Yang HL, Dong YB, Elliott MJ, Liu TJ, McMasters KM . Caspase activation and changes in Bcl-2 family member protein expression associated with E2F-1-mediated apoptosis in human esophageal cancer cells. Clin Cancer Res 2000; 6: 1579–1589.
Rogoff HA, Pickering MT, Frame FM, Debatis ME, Sanchez Y, Jones S et al. Apoptosis associated with deregulated E2F activity is dependent on E2F1 and Atm/Nbs1/Chk2. Mol Cell Biol 2004; 24: 2968–2977.
Kherrouche Z, Blais A, Ferreira E, De Launoit Y, Monte D . ASK-1 (apoptosis signal-regulating kinase 1) is a direct E2F target gene. Biochem J 2006; 396: 547–556.
Xie W, Jiang P, Miao L, Zhao Y, Zhimin Z, Qing L et al. Novel link between E2F1 and Smac/DIABLO: proapoptotic Smac/DIABLO is transcriptionally upregulated by E2F1. Nucleic Acids Res 2006; 34: 2046–2055.
Li JM, Hu PP, Shen X, Yu Y, Wang XF . E2F4-RB and E2F4-p107 complexes suppress gene expression by transforming growth factor beta through E2F binding sites. Proc Natl Acad Sci USA. 1997; 94: 4948–4953.
Spender LC, Inman GJ . TGF-beta induces growth arrest in Burkitt lymphoma cells via transcriptional repression of E2F-1. J Biol Chem 2009; 284: 1435–1442.
Lukas J, Petersen BO, Holm K, Bartek J, Helin K . Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4 A-mediated growth suppression. Mol Cell Biol 1996; 16: 1047–1057.
Itoh S, Ericsson J, Nishikawa J, Heldin CH, ten Dijke P . The transcriptional co-activator P/CAF potentiates TGF-beta/Smad signaling. Nucleic Acids Res 2000; 28: 4291–4298.
Nahle Z, Polakoff J, Davuluri RV, McCurrach ME, Jacobson MD, Narita M et al. Direct coupling of the cell cycle and cell death machinery by E2F. Nat Cell Biol 2002; 4: 859–864.
Ianari A, Natale T, Calo E, Ferretti E, Alesse E, Screpanti I et al. Proapoptotic function of the retinoblastoma tumor suppressor protein. Cancer Cell 2009; 15: 184–194.
Lin WC, Lin FT, Nevins JR . Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev 2001; 15: 1833–1844.
Martinez-Balbas MA, Bauer UM, Nielsen SJ, Brehm A, Kouzarides T . Regulation of E2F1 activity by acetylation. EMBO J 2000; 19: 662–671.
Wang B, Liu K, Lin FT, Lin WC . A role for 14-3-3 tau in E2F1 stabilization and DNA damage-induced apoptosis. J Biol Chem 2004; 279: 54140–54152.
Hofmann F, Martelli F, Livingston DM, Wang Z . The retinoblastoma gene product protects E2F-1 from degradation by the ubiquitin-proteasome pathway. Genes Dev 1996; 10: 2949–2959.
Laiho M, DeCaprio JA, Ludlow JW, Livingston DM, Massague J . Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell 1990; 62: 175–185.
Dick FA, Dyson N . pRB contains an E2F1-specific binding domain that allows E2F1-induced apoptosis to be regulated separately from other E2F activities. Mol Cell 2003; 12: 639–649.
Iavarone A, Massague J . E2F and histone deacetylase mediate transforming growth factor beta repression of cdc25A during keratinocyte cell cycle arrest. Mol Cell Biol 1999; 19: 916–922.
Chen CR, Kang Y, Siegel PM, Massague J . E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell 2002; 110: 19–32.
Frederick JP, Liberati NT, Waddell DS, Shi Y, Wang XF . Transforming growth factor beta-mediated transcriptional repression of c-myc is dependent on direct binding of Smad3 to a novel repressive Smad binding element. Mol Cell Biol 2004; 24: 2546–2559.
Acknowledgements
We thank Dr. Kristian Helin for kindly providing the mutant E2F1 expression vectors and Dr. Lili Yamasaki for generously providing wild-type and E2F1 knockout MEFs. This work was supported by grants from the Canadian Institutes for Health Research (CIHR, MOP-114904 to JJL). JJL is the recipient of the McGill Sir William Dawson Research Chair and JK holds a CIHR Frederick Banting and Charles Best Doctoral Research Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Stephanou
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Korah, J., Falah, N., Lacerte, A. et al. A transcriptionally active pRb–E2F1–P/CAF signaling pathway is central to TGFβ-mediated apoptosis. Cell Death Dis 3, e407 (2012). https://doi.org/10.1038/cddis.2012.146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2012.146
Keywords
This article is cited by
-
c-Myc shuttled by tumour-derived extracellular vesicles promotes lung bronchial cell proliferation through miR-19b and miR-92a
Cell Death & Disease (2019)
-
E2F1 and E2F7 differentially regulate KPNA2 to promote the development of gallbladder cancer
Oncogene (2019)
-
Transforming Growth Factor-beta Regulation of Ephrin Type-A Receptor 4 Signaling in Breast Cancer Cellular Migration
Scientific Reports (2017)
-
Autophagy and epithelial–mesenchymal transition: an intricate interplay in cancer
Cell Death & Disease (2016)
-
The leukemia inhibitory factor (LIF) and p21 mediate the TGFβ tumor suppressive effects in human cutaneous melanoma
BMC Cancer (2015)