Abstract
Exosomes are cell-derived vesicles that convey key elements with the potential to modulate intercellular communication. They are known to be secreted from all types of cells, and are crucial messengers that can regulate cellular processes by ‘trafficking’ molecules from cells of one tissue to another. The exosomal content has been shown to be broad, composed of different types of cytokines, growth factors, proteins, or nucleic acids. Besides messenger RNA (mRNA) they can also contain noncoding transcripts such as microRNAs (miRNAs), which are small endogenous cellular regulators of protein expression. In diseases such as cancer, exosomes can facilitate tumor progression by altering their vesicular content and supplying the tumor niche with molecules that favor the progression of oncogenic processes such as proliferation, invasion and metastasis, or even drug resistance. The packaging of their molecular content is known to be tissue specific, a fact that makes them interesting tools in clinical diagnostics and ideal candidates for biomarkers. In the current report, we describe the main properties of exosomes and explain their involvement in processes such as cell differentiation and cell death. Furthermore, we emphasize the need of developing patient-targeted treatments by applying the conceptualization of exosomal-derived miRNA-based therapeutics.
Similar content being viewed by others
Facts
-
Exosomes are key elements that facilitate intercellular communication; depending on their vesicular content (‘cargo’), they can modulate tumor cells by influencing major cellular pathways such as apoptosis, cell differentiation, angiogenesis and metastasis.
-
This communication can involve the exchange of molecules such as small noncoding RNAs (e.g. miRNAs) between malignant, nontransformed and stromal cells (in all directions).
-
Exosomal miRNAs represent ideal candidates for biomarkers, with multiple applications in the management of an array of pathologies such as cancer.
-
Manipulating exosomal miRNAs suggests new alternatives for patient-tailored individualized therapies.
Open Questions
-
What are the mechanisms through which exosomal contents (e.g., miRNA) are selected to be further secreted from tumor cells? Are these mechanisms similar/different when the secretion is from nontransformed or stromal cells? Are the miRNAs conveyed in exosomes a reflection of the cellular miRNA composition?
-
How are the molecules sequestered in exosomes influencing the cancer hallmarks (e.g., mediating immune evasion or establishing metastatic niches)?
In ancient Greek mythology, Hermes was the wing-shod messenger of the Olympians, the beloved son of Zeus and of the nymph, Maia. He was committed to numerous responsibilities given by Zeus, and the most important one was to serve as a link between two worlds, taking messages from the gods to mankind.1 By applying the wisdom of ancient philosophy to modern biomedical research, there is clear resemblance between the way the two worlds – mankind and gods – co-evolved, with the ways ontogenesis and oncogenesis are thought to develop: by communicating through messengers that for years were unknown to scientists.
The release of membrane-bound vesicles is a highly conserved biological event in prokaryotes and eukaryotes, a fact that attributes these vesicles an important role in regulating physiological cellular processes.2 Interestingly, recent studies have discovered that transformed-tumor cells can take advantage of these endogenous ‘trafficking systems’ by transferring molecules that activate cancer-related pathways such as anti-apoptotic, proliferative or other tumorigenic ones. Initially, malignant tumor cells develop and proliferate in their local niche through the activation of endogenous oncogenic proteins and pathways. However, after some time, these cells recruit endogenous systems such as vesicle secretion, to broaden communication within the local tumor microenvironment and beyond. For example, at the vascular interface they orchestrate the enrollment of endothelial, perivascular or inflammatory cells, as well as platelets and clotting factors to supply tumor requirements. Actions such as these lead to the disruption of the local vascular homeostasis and also to the alteration of vital pathways that can favor the development of a tumor microenvironment with metastatic potential.3, 4 Through their ‘trafficking’, membrane-bound vesicles transport ‘molecular machinery’ with the potential of causing physiological effects that can very well favor tumorigenesis. Several key elements have been shown to be sequestered and transported through these vesicles: cytokines, growth factors, proteins, lipids, messenger RNAs (mRNAs) or noncoding transcripts, including microRNAs (miRNAs).2, 3, 4, 5
MiRNAs are short single-stranded (19–25 nucleotides in length) nonprotein-coding RNA transcripts (ncRNA) that are initially produced in the nucleus and then transported into the cytoplasm, where they undergo a series of steps to acquire maturation. Mature miRNAs regulate gene expression by binding (through watsonian complementarity) to the sequence of a target mRNA. This interaction results in translational repression and/or mRNA cleavage, which consequently decreases the levels of the mRNA coding protein.6, 7 MiRNAs have been found to be aberrantly expressed in many diseases.8, 9, 10, 11, 12 For example, in cancer, the tumor microenvironment contains deregulated miRNA levels, and a reason for their altered levels is because they are being actively secreted as membrane-bound vesicular content.
Cells can secrete ‘molecular machinery’ through several types of vesicular carriers that are composed of both membrane and cytosolic constituents. These carriers have been shown to contain nuclear/cytoplasmic proteins, but also nucleic acids13 as well as other molecules. The biogenesis and main characteristics of these vesicular systems are presented in Table 1 and Figure 1a. Vesicular carriers can be classified based on their tissue of origin, their size and targeted function. In this manner, they can be categorized as either exosomes, microvesicles or apoptotic bodies.13, 14, 15 Out of the three, exosomes are currently studied the most. They are depicted as vesicular carriers involved in mediating both cell-to-cell communication16 and the trafficking of several molecules that are regarded as insoluble or classified as endogenous cellular components. Exosomes are usually transported through biological fluids such as plasma or serum. Thus, body fluids comprise a complex variety of extracellular vesicles that are being secreted from different tissues and therefore different cell types. The mechanisms regarding exosomal formation from their tissue of origin, their traveling through body fluids and their evolutionary significance has been studied in the past.7, 8 It is known that exosomes display a set of proteins that reflect their tissue of origin and more specifically their target cells.17 Furthermore, their trafficking is cell specific,18, 19 and the exosomal content of the vesicles is highly regulated by a group of proteins that together form the endosomal sorting complex required for transport (ESCRT).2, 3 This complex determines the relative abundance of proteins, mRNA,20 miRNAs (...and so on), that will be segregated into a vesicle to be further transported elsewhere.
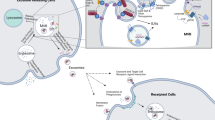
Exosome structure and function. (a) Exosome biogenesis and function: once released, exosomes can travel through biological fluids (e.g. serum, lymph) and furthermore, they can be internalized by cells from nearby tissues, or more so, reach body sites very far from their tissue of origin. (b) Main classes of molecules included in the exosomal cargo. MT, mitochondria; MVB, multivesicular bodies
In this review, we aim to emphasize the roles that exosomal miRNAs have as essential communicators between the cancer cells and all other cell types. We also describe the consequences of their ‘molecular trafficking’ in cancer development, progression and metastasis.
Exosomes, Their Synthesis and Internal Content
Exosomes are naturally produced, membrane vesicle-like structures that range in size between 40 and 100 nm.15 They have a very unique cup-shaped morphology when they are observed through transmission electron microscopy.16, 21 After being secreted, these structures have been described to have a buoyant density of 1.10 to 1.21 g/ml in sucrose. Aside from lipids such as cholesterol, ceramide or sphingolipids, their composition includes proteins like Rab GTPases, annexins or Tsg101, as well as integrins or tetraspanins (such as CD63, CD9 or CD82)8, 15, 22, 23 (Figure 1b).
Several experimental procedures have served as assessment tools to detect the presence, size, purity and content of exosomal structures. In general, exosomal content can be assessed by a wide range of approaches such as western blot, mass spectrometry, fluorescence-activated cell sorting (FACS) or immunoelectronic microscopy. These techniques have individually proven that exosomes contain a variety of lipids, hundreds of proteins, thousands of RNA species21 including over 500 different miRNAs.24, 25
Immediately after their synthesis,15 exosomes are released and can remain in the extracellular space near the cell they originated from. Alternatively, they can also travel through body fluids such as blood, urine, amniotic fluid, saliva, lung surfactant, malignant effusions or breast milk. The end result of this dynamic process is a variety of regulative molecules being transported to different tissues in different places, and influencing cellular processes. Exosomes have been shown to carry proteins,26 many of which have the potential to influence multiple regulatory mechanisms. For example, exosomes can transport annexins that have the ability of altering the dynamics of the cytoskeleton. They can also transport Rab GTPases that promote a wide range of cellular events regarding membrane fusions or endosomal sorting complexes desired for transport.26, 27, 28 In hematopoietic tissues, exosomes express high levels of MHC class I or II molecules on their surfaces as well as other types of adhesion molecules such as CD146, CD9, EGFRvIII, CD18, CD11a or LFA-3/CD58.29, 30, 31 Specific surface markers such as these allow exosomes to be uptaken even by distant cells with a high level of specificity. This mechanism facilitates the transfer of proteins, mRNAs, miRNAs, lipids or other types of molecules to different parts of the body, and thus in this way ‘exosome-trafficking’ becomes a type of particle-based endocrine system.
Exosomes as Key Determinants of Intercellular Communication
The release of cellular invaginations from the membrane into the extracellular space has been known and described by Black32 over three decades ago. Initially, exosomes were described as key players with important roles in cell-to-cell communication as well as key physiological and pathological processes.33 However, it was recently proven that vesicles released by cells could be indicative of the cell type and/or its function.2, 14 With the progression of exosomal-related studies, these vesicles have now become a useful tool with diagnostic utility, and a novel strategy to develop highly specific drug delivery systems.
Exosomes can contain a variety of differentiation factors that could be released into body fluids affecting key processes of cellular homeostasis and differentiation.18 The disruption of this homeostasis has important repercussions that may result in very different outcomes such as programmed cell death or, the contrary, chaotic cell proliferation and growth.34 A deregulated homeostatic environment can eventually lead to the development of several pathological conditions, including malignancies such as cancer. Thus, the contribution of exosomes in the altering of normal intercellular as well as intracellular signaling has been proven to be as significant, or even more so, as lysosomes or proteasomes.13
Adult tissues, more specifically the neoplastic ones, are heterogeneous complex systems composed of a variety of cell types that continuously interact with one another. Both normal and tumor cells typically secrete proteins, and thus different subtypes of exosomes can be responsible for carrying these proteins from cell to cell, resulting in a type of ‘message delivery’. In cancer, the ‘messages’ are highly dysregulated and therefore intracellular circuits are either disrupted or overwhelmed, resulting in tumor initiation and development. Exosomes are the link between disruption of homeostasis in normal tissues and the development of oncogenesis (because they influence crucial modulators of cell cycle, proliferation, apoptosis, etc).35, 36, 37, 38, 39
Exosomal miRNAs in Cell Death and Differentiation
Exosomes are important for various aspects of tumor biology, such as cell de-differentiation and programmed cell death. Yang et al.40 have proven that purified exosomes from the supernatant of T24 bladder cancer cells demonstrated to have overexpressed levels of Bcl-2 and cyclin D1, but reduced levels of Bax and caspase-3 proteins. This same research group also proved that there is a direct, proportional link between the amount of secreted exosomes and the expression of phosphorylated Akt and extracellular signal-regulated protein kinases (ERKs), both of which are crucial elements regulating apoptosis and cell differentiation processes. ‘Autocrine’ or ‘paracrine’ intercellular trafficking of proteins/genetic material via exosomes has proven to be extremely efficient for intercellular communication (specifically in tumors). For example, the release of exosomes may protect tumors from cell death, and surge the transmission of signals that may lead to an increase in cell proliferation.41 The fact that exosomes can tightly regulate cellular processes such as these, most certainly implies that these vesicles could be targeted therapeutically to induce apoptosis and cell differentiation or to inhibit proliferation.4
The physiological role of exosomes is still a controversial topic, although to this date increasing amounts of experimental data have implicated them in various key processes.28 Nevertheless, some studies are still considered speculative. For example, Turchinovich et al.42 suggested that circulating miRNAs are components or remaining parts of exosomes from dying cells that persist in the extracellular environment. This group proposed that those same miRNAs could act in a paracrine manner because exosomes supply a stable intravesicular environment for them as well as for other proteins that they require for their biogenesis and maturation (e.g. Ago2).42 Similarly, additional studies have demonstrated that molecules, but specifically miRNAs, embedded in exosomal vesicles are protected from RNAases in the blood because they are surrounded by a lipidic membrane.43 Thus, by increasing their stabilization and protecting them from degradation, exosomes enable molecules such as miRNAs to evade immunosurveillance mechanisms and thereby prevent events such as apoptosis. In this manner, exosomes allow an increase in pathological cancer-related processes such as angiogenesis, and even more, they confer tumors the ability of resisting chemo- and radiotherapy.43
Recently, it has been demonstrated that exosomes are uptaken in a selective way by neighbor cells but also by distant ones (including immune cells, through microenvironment cooperation), and that their uptake can lead to the activation of cell proliferation and differentiation.43, 44 They can contribute to these processes by embedding (as part of their ‘molecular cargo’) miRNAs that are able to reprogram multiple cellular mechanisms in recipient cells.44
Recent reports suggest that exosomes are implicated in facilitating tumors with immune-evasive strategies (a known cancer hallmark).45, 46 Previous literature has suggested that these processes may occur as an effect of the direct interactions of immune effector cells and cancer exosomes; however, several other molecular intermediates are thought to be contributing as well (although the exact detailed mechanisms are not completely understood).4, 5, 45, 46 Through predictions, it has been speculated that a group of exosomal derived-miRNAs (e.g., let-7, miR-17-92 cluster, miR-21, miR-25, miR-106, miR-155 or miR-200 family) participate as mediators that alter immune responses by regulating T cells.45, 46, 47 Further research aims to focus on deciphering not only which miRNAs mediate immune evasion, but also the mechanisms through which they do so (Figure 2).
The current direction regarding the study of exosomal miRNAs contributing to immune evasion, is to verify their vesicular content by recovering them from body fluids. With strategies such as reversing immune dysfunction clinicians could obtain an increase in the initial therapeutic response from patients, or alternatively, the reversal of therapeutic resistance, or relapse prevention.48 Exosomes or more specifically their vesicular content have already shown to target immunosuppressive pathways. For example, exosomal-derived miR-155 directly influences tissues and their microenvironments by causing an evasion of immune cell response.49 Its inhibition actually proved to sensitize CD4+ Th cells for TREG suppression.50 Therefore, miR-155 represents a primeval modulator of the immune system with significant clinical implications because it targets receptors/ligands requested for anticancer immunity. Thus, the delivery of anti-miR-155 through artificial exosomes could be considered a potential novel therapy.
Exosomal miRNAs in Cancer
Generally, tumor cells secrete a large amount of exosomes. Exosomal release has been proven to have prognostic relevance, where a higher amount of serum exosomes typically predicts unfavorable outcomes.12, 44, 45 In 2007, Valadi et al.51 made significant progress by proving that genetic materials, mRNAs and miRNAs, are indeed transported through exosomal trafficking of molecules. Further along, other studies confirmed the presence of miRNAs in microvesicles or exosomes.52 Several others have been published associating exosomal miRNAs and their influence in cancer. For example, glioblastoma-derived exosomes were proven to be able to transport actively translated mRNA to different target cells. Even more, these exosomes were capable of inducing proliferation and tumor growth.53 Similarly, in metastatic gastric cancer, the let-7 miRNA family (known to have a tumor-suppressive role by targeting oncogenes such as RAS and HMGA2) is selectively released through exosomes and this allows the targeting of oncogenic signals during metastasis development in the recipient tissue.54 For a detailed example/model of exosomal content contributing to metastasis in cancer cells, refer to Figure 3. In addition, tumor-derived exosomes may also transport proteins that inhibit normal cellular processes such as apoptosis. MiRNA transportation from tumor cells into the vascular system promotes a rapid method of exosome trafficking, which can promote processes such as disease spread/metastasis (refer to Figure 4).
Exosome secretion by tumor cells. The released exosomes are uptaken by neighboring cells and are capable of inducing pathways involved in cancer initiation/progression (e.g. migration, invasion), or alternatively, they can modulate immunogenic responses. Their downstream effect is attributed to their molecular cargo, which can be rich in miRNAs
Tumor-derived exosomes are secreted via a constitutive/inducible pathway that can be activated by a p53-mediated response. Through this pathway (and possibly additional ones that remain unknown), exosomes can transport microRNAs into the vascular system and regulate important cellular processes such as immunosurveillance, apoptosis and angiogenesis. For example: (a) TP53 can induce the activity of TSAP6 proteins, thereby modulating key functions associated with tumor-derived exosome trafficking. Exosomes are generated by reverse budding of the membrane of multivesicular bodies (MVBs) that fuse with the plasma membrane, causing the release of these particles outside the cell. (b) From this moment on, exosomes enter the vascular or lymphatic system and circulate freely until they bind to their specific target. (c) The release from the cell is possible after the membrane is stimulated by calcium ionophores or phorbol esters. Inositol 3-kinase inhibitors can inhibit this process
Interestingly, when cells are subjected to stressful conditions, miRNAs can promote tumor survival through an exosomal-mediated pathway, increasing the levels of oncogenes involved in survival and evasion of apoptosis.4 For example, in a study regarding cervical tumor cells, the secretion of survivin through exosomes was noticed after cells were exposed to radiation treatment. These mechanisms may be considered important for cancer cells to develop strategies that facilitate drug resistance.55 On a different study, proteins such as FasL or TRAIL were shown to be expressed on the surfaces of exosomes in melanoma and colorectal carcinomas.37 This has negative consequences on patient therapeutic responses, as they inhibit the ability of T cells to enable apoptosis. In this way exosomes can interfere with cellular pathways not only by trafficking specific molecules, but also by interacting with immune cells through surface–surface interactions.
To some extent, exosomes have been thought to set up an environment that could favor tumor development. Peinado et al.56 described exosomes as key elements in the assembly of the pre-metastatic niche. This last study presented for the first time the role of exosomes as part of the ‘seed and soil’ theory. Exosomes isolated from highly metastatic melanomas have been shown to increase the metastatic potential of primary tumors by facilitating (with their vesicular content) bone marrow progenitor cells to acquire a pro-metastatic phenotype via tyrosine kinase receptor activation.57 A more recent investigation reported that exosomal miR-105 enhances angiogenesis in a cancer context by targeting ZO-1 (a protein that in humans is encoded by the TJP1 gene).58 In this way exosomes target proteins related to cell junctions and increase epithelial cell permeability, thereby promoting tumor invasion.
Several studies have stated the importance of exosomal-derived miRNAs in determining the overall effect of (already known) chemotherapies in cancer patients. For example, in breast cancer, exosomes released by HER-2-positive cancer cells have their expression modulated by various growth factors that include EGF and heregulin, a pleiotropic molecule that binds to the ErbB receptor family. These are some of the most extensively studied HER-2 cross-activating ligands and are secreted in large quantities in the surrounding neoplastic niche. Through the expression of these receptors, exosomes manage to escape/inhibit the activity of trastuzumab, a previously established therapeutic strategy for this type of cancer.59 Furthermore, in this subtype of breast cancer, the HER-2 display on the surface of exosomes has been correlated with an increase in the binding of trastuzumab to exosome vesicles and not the original target (which are the cancer cells). This most definitely hinders therapeutic inefficiency.60 In addition to exosome-mediated mechanisms, the clinical resistance to herceptin therapy is also related to the cancer cells protecting themselves from NK-cell mediated antibody-dependent cytotoxicity (ADCC), which minimizes the chances of reducing tumor burden.61, 62 Thus, in this way exosomes confer cancer cells additional strategies that can allow them to overcome the effects of pre-existing therapeutics.
Exosomal contents such as miRNAs typically relate to the ones being expressed in the tissues from which they are secreted. However, in some cases the exosomal miRNA content does not correlate to miRNA profiles of a particular cell type.33 On occasions, tumor-derived exosomes establish a pre-metastatic niche which can regulate ‘premetastatic behaviors’ because of the miRNAs that have been transferred from other tissues of origin (Figure 4). For example, in 2008, Taylor and Gercel-Taylor31 observed circulating tumor microvesicles in the peripheral blood of patients diagnosed with ovarian cancer, and these vesicles contained specific ‘molecular cargo’ when compared to vesicles from healthy patients. This suggests that vesicular cargo such as miRNAs are different in their content when they are secreted from a tumor microenvironment versus when they originate from the exact same tissue, but from a healthy patient. Similarly, studies done in melanoma also described significant differences in mRNA and miRNA signatures when comparing exosomes derived from melanoma with the ones arising from normal melanocytes: the proteins such as HAPLN1, GRP78, syntenin-1 and annexin A1 were present in melanoma-derived exosomes, but not in normal melanocytes.63
Many oncogenes and processes promoting carcinogenesis (as well as other diseases) have been related to specific miRNAs and exosomes, and herein we present some examples. Exosomal-derived let-7 was proven to be involved in the modulation of Toll-like receptor 7.64 In addition, exosomal miR-21 and miR-29a were demonstrated to stimulate Toll-like receptor-7 and -8, causing a prometastatic inflammatory response and activating tumor growth and metastasis.65 Furthermore, Ferrajoli et al.66 described an overexpression of miR-155 in free circulating and blood microvesicles for MBL (monoclonal B-cell lymphocytosis) and CLL (chronic lymphocytic leukemia) patients. Separately, in another study, 11 exosomal miRNAs were observed overexpressed in prostate cancer patients and miR-141 and miR-375 were presented as potential markers of metastatic prostate cancer based on exosomal and microvesicle samples.67 More so, exosomal miRNA expression profiling proved that AZ-P7a cells (a metastatic gastric cancer cell line) release let-7 miRNAs via exosomes into the extracellular environment to maintain their oncogenesis.54 In addition, exosomal miR-223 was shown to be released by macrophages, and to be efficiently transferred to breast cancer cells, thereby targeting cellular invasion.68 Finally, it has already been reported that exosomes are able to transfer phenotypic characteristics from tumor cells to other cell types, conferring aggressive behaviors to receptor cells.69
Exosomes as Novel Cancer Biomarkers
Exosome-associated signatures from various malignancies are expected to predict the presence of early disease before a formal diagnosis is done through classic imaging techniques such as MRI, CT or a PET scan.70, 71, 72 In addition, these signatures might contribute by confirming or discarding the results of biomarkers that are nowadays considered standard of care, such as the prostate-specific antigen (PSA),73 carbohydrate antigen CA-90 or CA-125.74
Exosomes are intercellular carriers of many molecules and, thus, they may be considered as novel sources of unknown potential biomarkers.75 It would be very useful to develop new mechanisms to monitor the transcriptome expression pattern based on the use of noninvasive biological fluids.76, 77, 78 As the composition of nucleic acids (specifically RNA) reflect in most cases the nature and origin of the parental cell, exosomes may be considered valuable carriers of cell-specific information because their content provides an insight glance of the proteins being transcribed in the original tissue.79, 80, 81 In this manner, exosomes may be used in the clinic to achieve early diagnostics and prognosis information, but also to stratify and separate patients into different clinical categories in order to determine specific targeted therapies. With the increase in studies aiming to find selective methods to isolate and purify exosomes, these approaches seem hopeful and promising as researchers might actually find relevant biomarkers to diagnose specific diseases or to better define disease stages.16, 26
Various functional studies have proven that exosomes display a delimited cell tropism because they target certain cells or tissues by displaying receptors in the membranes (including malignant ones).82, 83 For example, urine-derived exosomes from prostate cancer patients display (continuously) specific molecular markers such as PCA-3 or TMPRSS2 which are tissue specific.84 In addition, exosomes are present in all bodily fluids, minimizing the need of performing invasive or potentially harmful diagnostic procedures (such as continuous rectal examinations for prostate cancer screening). These facts make them strong candidates for biomarkers of disease presence, progression or even therapeutic response.85, 86
Recent data promote the use of dendritic cell-derived or even tumor-derived exosomes to understand which therapies are more favorable to use in the clinic.87, 88 For more details regarding studies using exosomes for anticancer vaccines refer to Table 2. On a separate note, by analyzing the proteomic profile of exosomes isolated from neuroblastoma, Marimpietri et al.89 discovered that neuroblastoma cells secrete extracellular multivesicular bodies that express the GD2 disialoganglioside, a protein involved in cell immune response, cell differentiation, cell proliferation and progression. Thus, in this manner, proteomic profiles of exosomes could be used to improve prognosis predictions, and diagnostic accuracy but also to predict of potential disease relapse in patients receiving chemotherapy or radiation treatment. Interestingly, in chronic myeloid leukemia (CML), Mineo et al.90 showed that a co-evolution between endothelial cells and CML cells are essential for leukemia progression and resistance to therapy. This is possible because of the fact that K562 malignant cells secret growth factors and various miRNAs and transport these ‘endothelial inducing factors’ via exosomes. As a result, tube formation is stimulated, even when treating with imatinib, a tyrosine kinase inhibitor that targets the Philadelphia chromosome-positive (Ph+) myeloid leukemia cells. In this case, the development of angiogenesis was reported to regulate the progression and dissemination of this hematological malignancy. In cases such as these, exosomes provide an alternative strategy to target angiogenesis in order to potentially achieve an even better therapeutic response in these patients.
Several studies have already proven successful in identifying exosomes as biomarkers.66 For example, increased levels of exosomal miR-141 and miR-375 were capable of predicting metastasis or disease recurrence in prostate cancer.91 Exosomal miRNAs have shown distinct profiles in ovarian cancer versus benign ovarian tumors. Moreover, the lack of exosomal miRNA in normal controls has also been observed and proven useful to distinguish healthy patients from others who have the disease.92 Similarly, in a lung cancer study, significant differences in total exosomal RNA levels in patients versus controls was found;93 and a similarity between the circulating exosomal miRNA and the tumor-derived miRNA patterns for lung cancer patients was identified.94 Finally, miR-1246 was considerably elevated in exosomes from serum samples of esophageal squamous cell carcinoma (ESCC) patients, and this was determined to be a powerful independent risk factor for poor survival.95 All together, these data sets suggest that circulating exosomal miRNA would be extremely useful tools to develop novel cancer biomarkers (Table 3).
Exosome-Based Therapy
Exosomes may have significant contribution in drug delivery, based on the facts that they are considered natural carriers of multiple types of molecules, including nucleic acids. They have an ideal prototype to fit the role of ‘physiologic liposomes’ because of their nano-scaled size.96, 97 One potential application could be stimulating the body’s natural immune system to recognize and eliminate the neoplastic cells through exosomal-mediated recognition. These endogenous delivery systems can overcome the difficulties confronted when trying to use artificially created vesicles.20, 21 Based on this initial idea, several clinical trials have already started using exosomes with the aim of developing effective anticancer vaccines (Table 4). One of the first steps in developing them has been reducing their immunogenicity98 and increasing their half-life in the circulation, thereby assuring protection of their ‘therapeutic cargos’, particularly in the case of nucleic acids (miRNA or small interfering RNAs (siRNAs)).99, 100 Thus, exosomes are actively investigated as delivery systems of therapeutic nucleic acids structures, proteins or macromolecular drugs in immunotherapy,101, 102 cancer and neurological diseases.103, 104, 105, 106
Cho et al.75 published one of the first reports using engineered exosomes to express a specific cancer antigen that could generate an immune antitumor response. In their study, they demonstrated that artificially introduced exosomes have the ability to stimulate and shrink the tumor mass. To this date, liposomes are the predesigned carriers that have been proven to effectively deliver genetic material, drugs, cytokines, adjuvants or antigens into the body. However, nowadays, exosomes seem to be even better carriers because they can be introduced exogenously, but as they are ‘naturally endogenous vesicles’, they possess a high biosafety profile.97
Emerging progress in nanotechnology has allowed the development of novel exosome-based carriers that can be used in preclinical and clinical trials. In the past, nanoparticles that could mimic the physical characteristics of various viruses have shown to induce a very strong immune response when testing them as vaccines for similar clinical management.107 Exosomes are very similar to nanoparticles because they could express/present certain antigens on their surface; however, their toxicity is minimal or none. For example, Lee et al.108 used extracted exosomes from autologous cancers and described them as nano-sized particles enriched with specific antigens. After combining them with dendritic cells in vitro, a very strong anticancer effect was noticed in both cell cultures and in vivo models of primary and advanced metastatic malignancies.
The highest level of applicable therapeutics expected to be achieved is the use of exosome-based treatments where their content involves miRNAs that can be trafficked to specific tissue types to achieve the silencing of oncogenes (Table 4). In a recent study, Wahlgren et al.109 successfully delivered siRNAs using several types of human exosomes as delivery systems to mononuclear blood cells. Similarly, exosomes expressing let-7a were intravenously injected into a xenograft breast cancer model and the treatment resulted in significant antitumoral activity caused by the targeting EGFR receptors.96 Furthermore, some studies have suggested that exosomes themselves can become natural carriers of exogenous siRNA to human cells in vitro and in vivo.110 An example of this was seen when trying to knock down RAD51, a potential therapeutic target for tumor cells.111
Conclusions and Perspectives
In order to achieve early cancer detection, we are in need of discovering and validating better standard-of-care assays that can identify the presence of malignancies through noninvasive techniques. Discovering that exosomes mediate intercellular communication through the delivery of molecules (such as miRNAs and other nucleic acids) has underlined a particular interest for the use of these microvesicles as potential cancer biomarkers. As exosomes express specific markers on their surface, they are ideal candidates that could lead to more efficient management of diseases such as cancer. Major regulatory pathways such as cell differentiation, proliferation, survival or apoptosis have been demonstrated to be influenced by the transfer of genetic material, such as noncoding RNAs through these secreted vesicles. MiRNAs are an exosomal component that has one of the most significant functions in intercellular signaling and tumor progression. Through the dysregulation protein homeostasis miRNAs facilitate tumorigenesis by reinforcing every single one of the cancer hallmarks. Thus, techniques such as molecular profiling of exosome miRNA signatures will not only add molecular knowledge to our understanding of communication between malignant cells and microenvironment, but will also facilitate the introduction of novel cancer biomarkers and therapeutics.
Abbreviations
- CLL:
-
chronic lymphocytic leukemia
- CML:
-
chronic myeloid leukemia
- ESCC:
-
esophageal squamous cell carcinoma
- miRNA:
-
microRNA
- mRNA:
-
messenger RNA
- ncRNA:
-
noncoding RNA
- MBL:
-
monoclonal B-cell lymphocytosis
References
Brown NO . Hermes the Thief; the Evolution of a Myth. University of Wisconsin Press: Madison, 1947; vi pp 164.
Soekmadji C, Russell PJ, Nelson CC . Exosomes in prostate cancer: putting together the pieces of a puzzle. Cancers (Basel) 2013; 5: 1522–1544.
Oskarsson T, Batlle E, Massague J . Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell 2014; 14: 306–321.
Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 2011; 68: 2667–2688.
Zhang HG, Zhuang X, Sun D, Liu Y, Xiang X, Grizzle WE . Exosomes and immune surveillance of neoplastic lesions: a review. Biotech Histochem 2012; 87: 161–168.
Fabian MR, Sonenberg N, Filipowicz W . Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 2010; 79: 351–379.
Ambros V . MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell 2003; 113: 673–676.
Croce CM, Calin GA . miRNAs, cancer, and stem cell division. Cell 2005; 122: 6–7.
Spizzo R, Almeida MI, Colombatti A, Calin GA . Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene 2012; 31: 4577–4587.
Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci USA 2008; 105: 5166–5171.
Calin GA, Croce CM . MicroRNAs and chromosomal abnormalities in cancer cells. Oncogene 2006; 25: 6202–6210.
Calin GA, Croce CM . MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6: 857–866.
Rak J, Guha A . Extracellular vesicles–vehicles that spread cancer genes. Bioessays 2012; 34: 489–497.
Alderton GK . Metastasis. Exosomes drive premetastatic niche formation. Nat Rev Cancer 2012; 12: 447.
Thery C, Zitvogel L, Amigorena S . Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002; 2: 569–579.
Simons M, Raposo G . Exosomes–vesicular carriers for intercellular communication. Curr Opin Cell Biol 2009; 21: 575–581.
Simpson RJ, Lim JW, Moritz RL, Mathivanan S . Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics 2009; 6: 267–283.
Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA et al. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods 2001; 247 (1-2): 163–174.
Rana S, Malinowska K, Zoller M . Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia 2013; 15: 281–295.
Lotvall J, Valadi H . Cell to cell signalling via exosomes through esRNA. Cell Adh Migr 2007; 1: 156–158.
Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G . The biogenesis and functions of exosomes. Traffic 2002; 3: 321–330.
Thery C, Ostrowski M, Segura E . Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9: 581–593.
Lee Y, El Andaloussi S, Wood MJ . Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet 2012; 21 (R1): R125–R134.
Mathivanan S, Simpson RJ . ExoCarta: a compendium of exosomal proteins and RNA. Proteomics 2009; 9: 4997–5000.
Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 2013; 14: 319.
Pfeffer SR . Two Rabs for exosome release. Nat Cell Biol 2010; 12: 3–4.
Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12: 19–30 sup pp 1-13.
Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 1999; 147: 599–610.
Buschow SI, Nolte-'t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic 2009; 10: 1528–1542.
Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ . Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol 2000; 165: 1259–1265.
Taylor DD, Gercel-Taylor C . Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Semin Immunopathol 2011; 33: 441–454.
Black PH . Shedding from normal and cancer-cell surfaces. N Engl J Med 1980; 303: 1415–1416.
Pap E, Pallinger E, Pasztoi M, Falus A . Highlights of a new type of intercellular communication: microvesicle-based information transfer. Inflamm Res 2009; 58: 1–8.
Gu J, Qian H, Shen L, Zhang X, Zhu W, Huang L et al. Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-beta/Smad pathway. PLoS One 2012; 7: e52465.
Kahlert C, Kalluri R . Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl) 2013; 91: 431–437.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S et al. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ 2008; 15: 80–88.
Whiteside TL . Tumour-derived exosomes or microvesicles: another mechanism of tumour escape from the host immune system? Br J Cancer 2005; 92: 209–211.
Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 2001; 7: 297–303.
Yang L, Wu XH, Wang D, Luo CL, Chen LX . Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol Med Rep 2013; 8: 1272–1278.
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 2012; 19: 107–120.
Turchinovich A, Weiz L, Langheinz A, Burwinkel B . Characterization of extracellular circulating microRNA. Nucleic Acids Res 2011; 39: 7223–7233.
Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM . Exosomes: fit to deliver small RNA. Commun Integr Biol 2010; 3: 447–450.
Turchinovich A, Weiz L, Burwinkel B . Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci 2012; 37: 460–465.
Clayton A, Mason MD . Exosomes in tumour immunity. Curr Oncol 2009; 16: 46–49.
Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol 2006; 176: 1375–1385.
Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 2014; 41: 89–103.
Marleau AM, Chen CS, Joyce JA, Tullis RH . Exosome removal as a therapeutic adjuvant in cancer. J Transl Med 2012; 10: 134.
Vigorito E, Kohlhaas S, Lu D, Leyland R . miR-155: an ancient regulator of the immune system. Immunol Rev 2013; 253: 146–157.
Stahl HF, Fauti T, Ullrich N, Bopp T, Kubach J, Rust W et al. miR-155 inhibition sensitizes CD4+ Th cells for TREG mediated suppression. PLoS One 2009; 4: e7158.
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO . Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008; 105: 10513–10518.
Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008; 10: 1470–1476.
Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One 2010; 5: e13247.
Khan S, Jutzy JM, Aspe JR, McGregor DW, Neidigh JW, Wall NR . Survivin is released from cancer cells via exosomes. Apoptosis 2011; 16: 1–12.
Peinado H, Lavotshkin S, Lyden D . The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol 2011; 21: 139–146.
Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18: 883–891.
Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014; 25: 501–515.
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol 2012; 227: 658–667.
Abdel-Razeq H, Marei L . Current neoadjuvant treatment options for HER2-positive breast cancer. Biologics 2011; 5: 87–94.
Nahta R, Esteva FJ . HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res 2006; 8: 215.
Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ . Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 2006; 3: 269–280.
Xiao D, Ohlendorf J, Chen Y, Taylor DD, Rai SN, Waigel S et al. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One 2012; 7: e46874.
Lehmann SM, Kruger C, Park B, Derkow K, Rosenberger K, Baumgart J et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci 2012; 15: 827–835.
Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA 2012; 109: E2110–E2116.
Ferrajoli A, Shanafelt TD, Ivan C, Shimizu M, Rabe KG, Nouraee N et al. Prognostic value of miR-155 in individuals with monoclonal B-cell lymphocytosis and patients with B chronic lymphocytic leukemia. Blood 2013; 122: 1891–1899.
Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B et al. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer 2012; 106: 768–774.
Yang M, Chen J, Su F, Yu B, Su F, Lin L et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer 2011; 10: 117.
O'Brien K, Rani S, Corcoran C, Wallace R, Hughes L, Friel AM et al. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur J Cancer 2013; 49: 1845–1859.
Paidpally V, Chirindel A, Lam S, Agrawal N, Quon H, Subramaniam RM . FDG-PET/CT imaging biomarkers in head and neck squamous cell carcinoma. Imaging Med 2012; 4: 633–647.
Wu C, Li F, Niu G, Chen X . PET imaging of inflammation biomarkers. Theranostics 2013; 3: 448–466.
Anderson NG, Butler AP . Clinical applications of spectral molecular imaging: potential and challenges. Contrast Media Mol Imaging 2014; 9: 3–12.
Felgueiras J, Silva JV, Fardilha M . Prostate cancer: the need for biomarkers and new therapeutic targets. J Zhejiang Univ Sci B 2014; 15: 16–42.
Yang H, Zhu L, Wang S, Lang J, Xu T . Noninvasive diagnosis of moderate to severe endometriosis: the platelet-lymphocyte ratio cannot be a neoadjuvant biomarker for serum cancer antigen 125. J Minim Invasive Gynecol 2013 e-pub ahead of print 10 July 2013; doi:10.1016/j.jmig.2013.06.003.
Cho JA, Park H, Lim EH, Lee KW . Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol 2012; 40: 130–138.
Sharma P, Sahni NS, Tibshirani R, Skaane P, Urdal P, Berghagen H et al. Early detection of breast cancer based on gene-expression patterns in peripheral blood cells. Breast Cancer Res 2005; 7: R634–R644.
Aaroe J, Lindahl T, Dumeaux V, Saebo S, Tobin D, Hagen N et al. Gene expression profiling of peripheral blood cells for early detection of breast cancer. Breast Cancer Res 2010; 12: R7.
Showe MK, Vachani A, Kossenkov AV, Yousef M, Nichols C, Nikonova EV et al. Gene expression profiles in peripheral blood mononuclear cells can distinguish patients with non-small cell lung cancer from patients with nonmalignant lung disease. Cancer Res. 2009; 69: 9202–9210.
Chaput N, Taieb J, Schartz NE, Andre F, Angevin E, Zitvogel L . Exosome-based immunotherapy. Cancer Immunol Immunother 2004; 53: 234–239.
Hao S, Bai O, Yuan J, Qureshi M, Xiang J . Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol 2006; 3: 205–211.
Viaud S, Terme M, Flament C, Taieb J, Andre F, Novault S et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS One 2009; 4: e4942.
Chaput N, Schartz NE, Andre F, Taieb J, Novault S, Bonnaventure P et al. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol 2004; 172: 2137–2146.
Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q et al. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol 2006; 36: 1598–1607.
Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer 2009; 100: 1603–1607.
Pant S, Hilton H, Burczynski ME . The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol 2012; 83: 1484–1494.
Bu N, Li QL, Feng Q, Sun BZ . Immune protection effect of exosomes against attack of L1210 tumor cells. Leuk Lymphoma 2006; 47: 913–918.
Guo F, Chang CK, Fan HH, Nie XX, Ren YN, Liu YY et al. Anti-tumour effects of exosomes in combination with cyclophosphamide and polyinosinic-polycytidylic acid. J Int Med Res 2008; 36: 1342–1353.
Xiu F, Cai Z, Yang Y, Wang X, Wang J, Cao X . Surface anchorage of superantigen SEA promotes induction of specific antitumor immune response by tumor-derived exosomes. J Mol Med (Berl) 2007; 85: 511–521.
Marimpietri D, Petretto A, Raffaghello L, Pezzolo A, Gagliani C, Tacchetti C et al. Proteome profiling of neuroblastoma-derived exosomes reveal the expression of proteins potentially involved in tumor progression. PLoS One 2013; 8: e75054.
Mineo M, Garfield SH, Taverna S, Flugy A, De Leo G, Alessandro R et al. Exosomes released by K562 chronic myeloid leukemia cells promote angiogenesis in a Src-dependent fashion. Angiogenesis 2012; 15: 33–45.
Tiryakioglu D, Bilgin E, Holdenrieder S, Dalay N, Gezer U . miR-141 and miR-375 induction and release are different from PSA mRNA and PCA3 upon androgen stimulation of LNCaP cells. Biomed Rep 2013; 1: 802–806.
Taylor DD, Gercel-Taylor C . MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008; 110: 13–21.
Rosell R, Wei J, Taron M . Circulating microRNA signatures of tumor-derived exosomes for early diagnosis of non-small-cell lung cancer. Clin Lung Cancer 2009; 10: 8–9.
Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH . Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer 2009; 10: 42–46.
Takeshita N, Hoshino I, Mori M, Akutsu Y, Hanari N, Yoneyama Y et al. Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Br J Cancer 2013; 108: 644–652.
Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 2013; 21: 185–191.
EL Andaloussi S, Mager I, Breakefield XO, Wood MJ . Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 2013; 12: 347–357.
Amigorena S . Anti-tumour immunotherapy using dendritic-cell-derived exosomes. Res Immunol 1998; 149 (7-8): 661–662.
Hu G, Yao H, Chaudhuri AD, Duan M, Yelamanchili SV, Wen H et al. Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell Death Dis 2012; 3: e381.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ . Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011; 29: 341–345.
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 1998; 4: 594–600.
Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet 2002; 360: 295–305.
Ling H, Fabbri M, Calin GA . MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov 2013; 12: 847–865.
Martinez MC, Andriantsitohaina R . Microparticles in angiogenesis: therapeutic potential. Circ Res 2011; 109: 110–119.
Patel S, Mehta-Damani A, Shu H, Le Pecq JB . An analysis of variability in the manufacturing of dexosomes: implications for development of an autologous therapy. Biotechnol Bioeng 2005; 92: 238–249.
Le Pecq JB . Dexosomes as a therapeutic cancer vaccine: from bench to bedside. Blood Cells Mol Dis 2005; 35: 129–135.
Bachmann MF, Jennings GT . Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 2010; 10: 787–796.
Lee EY, Park KS, Yoon YJ, Lee J, Moon HG, Jang SC et al. Therapeutic effects of autologous tumor-derived nanovesicles on melanoma growth and metastasis. PLoS One 2012; 7: e33330.
Wahlgren J, De LKT, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res 2012; 40: e130.
El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc 2012; 7: 2112–2126.
Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV . Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal 2013; 11: 88.
Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A . Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer 2009; 125: 1016–1026.
Ristorcelli E, Beraud E, Verrando P, Villard C, Lafitte D, Sbarra V et al. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J 2008; 22: 3358–3369.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ . Delivery of siRNA the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011; 29: 341–345.
Acknowledgements
Dr. Berindan-Neagoe’s work was financed by POSCCE 709/2010 grant with title: ‘Clinical and economic impact of proteome and transcriptome molecular profiling in neoadjuvant therapy of triple negative breast cancer (BREASTIMPACT)’. Dr. Calin is The Alan M Gewirtz Leukemia & Lymphoma Society Scholar. Work in Dr. Calin’s laboratory is supported in part by the NIH/NCI grants 1UH2TR00943-01 and 1R01 CA182905-01, Developmental Research Awards in Prostate Cancer, Multiple Myeloma, Leukemia (P50 CA100632) and Head and Neck (P50 CA097007) SPOREs, a SINF MDACC_DKFZ grant in CLL, a SINF grant in colon cancer, a Kidney Cancer Pilot Project, the Duncan Family Institutional Seed Funds, The Blanton-Davis Ovarian Cancer-2013 Sprint for Life Research Award, the Laura and John Arnold Foundation, the RGK Foundation, the Estate of CG Johnson, Jr. and by the CLL Global Research Foundation. The authors would like to thank Lucian Craciun for the assistance with the figures.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by RA Knight
Rights and permissions
About this article
Cite this article
Braicu, C., Tomuleasa, C., Monroig, P. et al. Exosomes as divine messengers: are they the Hermes of modern molecular oncology?. Cell Death Differ 22, 34–45 (2015). https://doi.org/10.1038/cdd.2014.130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cdd.2014.130
This article is cited by
-
Characterization of circSCL38A1 as a novel oncogene in bladder cancer via targeting ILF3/TGF-β2 signaling axis
Cell Death & Disease (2023)
-
Extracellular vesicles from biological fluids as potential markers in castration resistant prostate cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
Exosomes from bone marrow-derived mesenchymal stem cells facilitate corneal wound healing via regulating the p44/42 MAPK pathway
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)
-
Roles and clinical application of exosomal circRNAs in the diagnosis and treatment of malignant tumors
Journal of Translational Medicine (2022)
-
The extracellular matrix alteration, implication in modulation of drug resistance mechanism: friends or foes?
Journal of Experimental & Clinical Cancer Research (2022)