Abstract
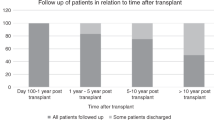
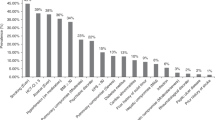
Despite international guidelines, optimal delivery models of late effects (LE) services for HSCT patients are unclear from the clinical, organizational and economic viewpoints. To scope current LE service delivery models within the UK NHS (National Health Service), in 2014, we surveyed the 27 adult allogeneic HSCT centres using a 30-question online tool, achieving a 100% response rate. Most LE services were led and delivered by senior physicians (>80% centres). Follow-up was usually provided in a dedicated allograft or LE clinic for the first year (>90% centres), but thereafter attrition meant that only ~50% of patients were followed after 5 years. Most centres (69%) had a standard operating procedure for long-term monitoring but access to a LE Multi-Disciplinary Team was rare (19% centres). Access to medical specialities necessary for LE management was good, but specialist interest in long-term HSCT complications was uncommon. Some screening (endocrinopathy, cardiovascular) was near universal, but other areas were more limited (mammography, cervical smears). Funding of extra staff and investigations were the most commonly perceived barriers to implementation of LE services. This survey shows variation in the long-term follow-up of allogeneic HSCT survivors within the UK NHS and further work is warranted to optimize effective, sustainable and affordable models of LE service delivery among this group.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA . Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet 1968; 2: 1366–1369.
National Marrow Donor Programme. Media fact sheet: 1 Million Blood Stem Cell Transplants Worldwide. Available at http://www.wbmt.org/fileadmin/pdf/01_General/One_Million_Transplants_Fact_Sheet_FINAL.pdf. Accessed 5 January 2016.
Farag SS, Maharry K, Zhang MJ, Pérez WS, George SL, Mrózek K et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60-70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant 2011; 17: 1796–1803.
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Preface. Bone Marrow Transplant 2009; 44: 453–455.
Center for International Blood and Marrow Transplant Research (CIBMTR); National Marrow Donor Program (NMDP); European Blood and Marrow Transplant Group (EBMT); American Society of Blood and Marrow Transplantation (ASBMT); Canadian Blood and Marrow Transplant Group (CBMTG); Infectious Disease Society of America (IDSA); Society for Healthcare Epidemiology of America (SHEA); Association of Medical Microbiology and Infectious Diseases Canada (AMMI); Centers for Disease Control and Prevention (CDC). Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Bone Marrow Transplant 2009; 44: 453–558.
Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2012; 18: 1150–1163.
Saraf SL, Oh AL, Patel PR, Jalundhwala Y, Sweiss K, Koshy M et al. Nonmyeloablative stem cell transplantation with alemtuzumab/low-dose irradiation to cure and improve the quality of life of adults with sickle cell disease. Biol Blood Marrow Transplant 2016; 22: 441–448.
Kekre N, Antin JH . Hematopoietic stem cell transplantation donor sources in the 21st century: choosing the ideal donor when a perfect match does not exist. Blood 2014; 124: 334–343.
Wingard JR, Majhail NS, Brazauskas R, Wang Z, Sobocinski KA, Jacobsohn D et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol 2011; 29: 2230–2239.
Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG et al. Late morality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood 2007; 110: 3784–3792.
Tichelli A, Bucher C, Rovó A, Stussi G, Stern M, Paulussen M et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood 2007; 110: 3463–3471.
DeFilipp Z, Duarte RF, Snowden JA, Majhail NS, Greenfield DM, Miranda JL et al. Metabolic syndrome and cardiovascular disease after hematopoietic cell transplantation: screening and preventive practice recommendations from the CIBMTR and EBMT. Biol Blood Marrow Transplant 2016; 22: 1493–1503.
Majhail NS . Secondary cancers following allogeneic haematopoietic cell transplantation in adults. Br J Haematol 2011; 154: 301–310.
Sun CL, Kersey JH, Francisco L, Armenian SH, Baker KS, Weisdorf DJ et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biol Blood Marrow Transplant 2013; 19: 1073–1080.
Hefner J, Kapp M, Drebinger K, Dannenmann A, Einsele H, Grigoleit GU et al. High prevalence of distress in patients after allogeneic hematopoietic SCT: fear of progression is associated with a younger age. Bone Marrow Transplant 2014; 49: 581–584.
Norkin M, Hsu JW, Wingard JR . Quality of life, social challenges, and psychosocial support for long-term survivors after allogeneic hematopoietic stem-cell transplantation. Semin Hematol 2012; 49: 104–109.
Rizzo JD, Wingard JR, Tichelli A, Lee SJ, Van Lint MT, Burns LJ et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, Center for International Blood and Marrow Transplant Research, and the American Society for Blood and Marrow Transplantation (EBMT/CIBMTR/ASBMT). Bone Marrow Transplant 2006; 37: 249–261.
FACT-JACIE. FACT-JACIE International Standards for Hematopoietic Cellular Therapy Product Collection, Processing, and Administration, 6th Edn, 2015. http://www.jacie.org/standards.
Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 348–371.
Syrjala KL, Martin PJ, Lee SJ . Delivering care to long-term adult survivors of hematopoietic cell transplantation. J Clin Oncol 2012; 30: 3746–3751.
Hashmi S, Carpenter P, Khera N, Tichelli A, Savani BN . Lost in transition: the essential need for need for long-term follow-up clinic or blood and marrow transplantation survivors. Biol Blood Marrow Transplant 2015; 21: 225–232.
Prepared by NHS England Specialised Services Clinical Reference Group for Blood and Marrow Transplantation. Clinical Commissioning Policy: Haematopoietic Stem Cell Transplantation (HSCT) (All Ages): Revised 2015. Reference: NHS England B04/P/a.
Crawley C, Kirkland K, Pearce R . BSBMT 7th report to specialist commissioners: the outcome of haematopoietic stem cell transplantation: an analysis of registry data for UK transplants performed 2008-2013 inclusive and a detailed analysis of transplant activity and outcomes in 2014, British Society of Blood and Marrow Transplantation, 2016.
NHS England. Service Specification No. 24 NHS Breast Screening Programme, 2016. https://www.england.nhs.uk/commissioning/pub-hlth-res/.
NHS England. Service Specification No. 25 NHS Cervical Screening Programme. 2016. Available at: https://www.england.nhs.uk/commissioning/pub-hlth-res/.
Miller PDE, de Silva T, Skinner R, Gilleece M, Peniket A, Hamblin A et al. Routine vaccination practice after adult and paediatric allogeneic haematopoietic stem cell transplant: a survey of UK NHS programmes. Bone Marrow Transplant 2017 (e-pub ahead of print 23 January 2017; doi:10.1038/bmt.2016.362).
Majhail NS, Rizzo JD . Surviving the cure: long term follow up of hematopoietic cell transplant recipients. Bone Marrow Transplant 2013; 48: 1145–1151.
Bhatia S . Caring for the long-term survivor after allogeneic stem cell transplantation. Hematology Am Soc Hematol Educ Program 2014; 2014: 495–503.
Mosher CE, DuHamel KN, Rini CM, Li Y, Isola L, Labay L et al. Barriers to mental health service use among hematopoietic SCT survivors. Bone Marrow Transplant 2010; 45: 570–579.
Shanklin VE, Snowden JA, Greenfield DM . Late treatment effects following bone marrow transplant: efficacy of implementing international guidelines. Eur J Cancer Care 2017 (e-pub ahead of print 27 December 2016; doi:10.1111/ecc.12623).
Greenfield DM, Absolom K, Eiser C, Walters SJ, Michel G, Hancock BW et al. Follow-up care for cancer survivors: the views of clinicians. Br J Cancer 2009; 101: 568–574.
Absolom K, Eiser C, Michel G, Walters SJ, Hancock BW, Coleman RE et al. Follow-up care for cancer survivors: the views of the younger adult. Br J Cancer 2009; 101: 561–577.
Absolom K, Eiser C, Turner L, Ledger W, Ross R, Davies H et al. Ovarian failure following cancer treatment: current management and quality of life. Hum Reprod 2008; 23: 2506–2512.
Hwang JP, Roundtree AK, Giralt SA, Suarez-Almazor M . Late effects and healthcare needs of survivors of allogeneic stem cell transplantation: a qualitative study. BMJ Support Palliat Care 2012; 2: 344–350.
Acknowledgements
We acknowledge the contribution of both British Society of Blood and Marrow Transplantation staff and personnel in the 27 UK adult allogeneic HSCT centres in the execution of this survey. We also specifically thank Rachel Pearce and Julia Perry, BSBMT Registry, for their assistance in providing the registry data.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Supplementary information
Rights and permissions
About this article
Cite this article
Hamblin, A., Greenfield, D., Gilleece, M. et al. Provision of long-term monitoring and late effects services following adult allogeneic haematopoietic stem cell transplant: a survey of UK NHS-based programmes. Bone Marrow Transplant 52, 889–894 (2017). https://doi.org/10.1038/bmt.2017.67
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.67
This article is cited by
-
The lived experience of long-term follow-up clinical care for haematopoietic stem cell recipients in England: a qualitative exploration
Journal of Cancer Survivorship (2023)
-
Longitudinal proteomics study of serum changes after allogeneic HSCT reveals potential markers of metabolic complications related to aGvHD
Scientific Reports (2022)
-
Survivorship care for allogeneic transplant patients in the UK NHS: changes centre practice, impact of health service policy and JACIE accreditation over 5 years
Bone Marrow Transplantation (2021)
-
Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors
Bone Marrow Transplantation (2020)