Abstract
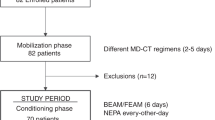
The aim of this study was to determine the efficacy of palonosetron combined with dexamethasone in prevention of chemotherapy (CT)-induced nausea and vomiting (CINV) in patients receiving high-dose (HD)-CT with auto-SCT, and the efficacy of a second dose of palonosetron in treating breakthrough emesis. One hundred thirty-four patients treated with HD-CT and auto-SCT for hematologic malignancies received palonosetron as prophylaxis for CINV on the first day of conditioning; patients were also administered dexamethasone throughout the entire period of conditioning. If breakthrough emesis occurred, a second dose of palonosetron was administered at 72 h after the first administration. Complete response and complete protection were observed in 36 and 26% of patients, respectively. One-half of the patients, re-treated with palonosetron for breakthrough emesis, were successfully rescued. Treatment with palonosetron plus dexamethasone seems to be encouraging in terms of prophylaxis of CINV and treatment of breakthrough emesis in the setting of HD-CT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 1997; 15: 103–109.
Osoba D, Zee B, Pater J, Warr D, Latreille J, Kaizer L . Determinants of postchemotherapy nausea and vomiting in patient with cancer. J Clin Oncol 1997; 15: 116–123.
Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW et al. Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer 2005; 13: 219–227.
Osoba D, Zee B, Warr D, Latreille J, Kaizer L, Pater J . Effect of post-chemotherapy nausea and vomiting on health related quality of life. Support Care Cancer 1997; 5: 307–313.
Roila F, Tonato M, Cognetti F, Cortesi E, Favalli G, Marangolo M et al. Prevention of DHAP-induced emesis: a double-blind multicenter randomized crossover study comparing ondansetron and ondansetron plus DHAP. J Clin Oncol 1991; 9: 675–678.
Latreille J, Stewart D, Laberge F, Hoskins P, Rusthoven J, McMurtrie E et al. DHAP improves the efficacy of granisetron in the first 24 h following high-dose DHAP chemotherapy. Support Care Cancer 1995; 3: 307–312.
Carmichael J, Bessell EM, Harris AL, Hutcheon AW, Dawes PJ, Daniels S et al. Comparison of granisetron alone and granisetron plus DHAP in the prophylaxis of cytotoxic-induced emesis. Br J Cancer 1994; 70: 1161–1164.
The Italian Group for Antiemetic Research. DHAP, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. N Engl J Med 1995; 332: 1–5.
The Italian Group for Antiemetic Research. Ondansetron vs metoclopramide, both combined with DHAP, in the prevention of DHAP-induced delayed emesis. J Clin Oncol 1997; 15: 124–130.
Aapro MS, Thuerlimann B, Sessa C, De Pree C, Bernhard J, Maibach R, Swiss Group for Clinical Cancer Research. A randomized double-blind trial to compare the clinical efficacy of granisetron with metoclopramide, both combined with DHAP in the prophylaxis of chemotherapy-induced delayed emesis. Ann Oncol 2003; 14: 291–297.
Pater JL, Lofters WS, Zee B, Dempsey E, Walde D, Moquin JP . The role of the 5-HT3 antagonist ondansetron and dolasetron in the control of delayed onset nausea and vomiting in patients receiving moderately emetogenic chemotherapy. Ann Oncol 1997; 8: 181–185.
Italian Group for Antiemetic Research. DHAP alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. N Engl J Med 2000; 342: 1554–1559.
Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 2003; 14: 1570–1577.
Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single dose trial vs dolasetron. Cancer 2003; 98: 2473–2482.
Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following higly emetogenic chemotherapy. Ann Oncol 2006; 17: 1441–1449.
Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced, nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose DHAP—The aprepitant protocol 052 study group. J Clin Oncol 2003; 21: 4112–4119.
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of the chemotherapy-induced nausea and vomiting. Cancer 2003; 97: 3090–3098.
Schmoll HJ, Aapro MS, Poli-Bigelli S, Kim HK, Park K, Jordan K et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with DHAP, for antiemetic efficacy in high-dose DHAP treatment. Ann Oncol 2006; 17: 1000–1006.
Warr DG, Hesketh PJ, Gralla RJ, Muss HB, Herrstedt J, Eisenberg PD et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 2005; 23: 2822–5851.
Sledge Jr GW, Einhorn L, Nagy C, House K . Phase III double-blind comparison of i.v. ondansetron and metoclopramide for patients receiving multiple-day DHAP based chemotherapy. Cancer 1992; 70: 2524–2528.
Noble A, Bremer K, Goedhals L, Cupissol D, Dilly SG . A double-blind, randomised crossover comparison of granisetron and ondansetron in 5-day fractionated chemotherapy: assessment of efficacy, safety, and patient preference. The Granisetron Study Group. Eur J Cancer 1994; 30A: 1083–1088.
Fox SM, Einhorn LH, Cox E, Powell N, Abdy A . Ondansetron vs ondansetron, DHAP, and chlopromazine in the prevention of nausea and vomiting associated with multiple-day DHAP chemotherapy. J Clin Oncol 1993; 11: 2391–2395.
Fauser AA, Pizzocaro G, Schueller J, Khayat D, Wilkinson P . A double-blind, randomised, parallel study comparing i.v. dolasetron plus DHAP and i.v. dolasetron alone for the management of fractionated DHAP-related nausea and vomiting. Support Care Cancer 2000; 8: 49–54.
Viner CV, Selby PJ, Zulian GB, Gore ME, Butcher ME, Wootton CM et al. Ondansetron—a new safe and effective antiemetic in patient receiving high-dose melphalan. Cancer Chemother Pharmacol 1990; 25: 449–453.
Perez EA, Tiemeier T, Solberg LA . Antiemetic therapy for high-dose chemotherapy with transplantation: report of a retrospective analysis of a 5HT3 regimen and literature review. Support Care Cancer 1999; 7: 414–424.
Abbott B, Ippoliti C, Bruton J, Neumann J, Whaley R, Champlin R . Antiemetic efficacy of granisetron plus DHAP in BM transplant patients receiving chemotherapy and TBI. BM Transplant 1999; 23: 265–269.
Ballen KK, Hesketh AM, Heyes C, Becker PS, Emmons RV, Fogarty K et al. Prospective evaluation of antiemetic outcome following high-dose chemotherapy with hematopoietic stem cell support. BM Transplant 2001; 28: 1061–1066.
Einhorn LH, Brames MJ, Dreicer R, Nichols CR, Cullen Jr MT, Bubalo J . Palonosetron plus DHAP for prevention of chemotherapy-induced nausea and vomiting in patients receiving multiple-day DHAP chemotherapy for germ cell cancer. Support Care Cancer 2007; 15: 1293–1300.
Musso M, Scalone R, Bonanno V, Crescimanno A, Polizzi V, Porretto F et al. Palonosetron (Aloxi) and DHAP for prevention of acute and delayed nausea and vomiting in patients receiving multiple-day chemotherapy. Support Care Cancer 2009; 17: 205–209.
Wong EH, Clark R, Leung E, Loury D, Bonhaus DW, Jakeman L et al. The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol 1995; 114: 851–859.
Navari RM . Prevention of emesis from multiple-day and high-dose chemotherapy regimens. J Natl Compr Canc Netw 2007; 5: 51–59.
Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 2006; 24: 2932–2947.
The Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC). Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 2006; 17: 20–28.
López-Jiménez J, Martín-Ballesteros E, Sureda A, Uralburu C, Lorenzo I, del Campo R et al. Chemotherapy-induced nausea and vomiting in acute leukemia and stem cell transplant patients: results of a multicenter, observational study. Haematologica 2006; 91: 84–91.
Rojas C, Stathis M, Thomas AG, Massuda EB, Alt J, Zhang J et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analag 2008; 107: 469–478.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Musso, M., Scalone, R., Crescimanno, A. et al. Palonosetron and dexamethasone for prevention of nausea and vomiting in patients receiving high-dose chemotherapy with auto-SCT. Bone Marrow Transplant 45, 123–127 (2010). https://doi.org/10.1038/bmt.2009.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.114
Keywords
This article is cited by
-
Netupitant/palonosetron without dexamethasone for preventing nausea and vomiting in patients with multiple myeloma receiving high-dose melphalan for autologous stem cell transplantation: a single-center experience
Supportive Care in Cancer (2022)
-
Prevention of chemotherapy-induced nausea and vomiting after high-dose melphalan and stem cell transplantation: review of the evidence and suggestions
Supportive Care in Cancer (2019)
-
2016 updated MASCC/ESMO consensus recommendations: prevention of nausea and vomiting following multiple-day chemotherapy, high-dose chemotherapy, and breakthrough nausea and vomiting
Supportive Care in Cancer (2017)
-
Palonosetron, aprepitant, and dexamethasone for prevention of nausea and vomiting after high-dose melphalan in autologous transplantation for multiple myeloma: A phase II study
International Journal of Hematology (2017)
-
A randomized trial of olanzapine versus palonosetron versus infused ondansetron for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients undergoing hematopoietic stem cell transplantation
Supportive Care in Cancer (2017)