Abstract
Background:
To investigate the issue of timing of radiation therapy (RT) after lumpectomy in relation to recurrences and outcome.
Methods:
Analysis was done on 1107 breast-conserving therapies (BCT) with 1070 women, all without lymph node metastasis and without any adjuvant systemic therapy. Timing was defined as time from lumpectomy till RT. Patients were categorised into tertiles: <45 days, 45–56 days, and 57–112 days.
Results:
Local control did not show a difference between the tertiles. The distant metastasis-free survival as well as the disease-specific survival showed a decreased outcome starting the RT to early after the lumpectomy.
Conclusion:
The results of this cohort study further refines the hypothesis that timing of RT in BCT might have an impact on outcome. It suggests that a randomised trial in timing of RT in BCT seems necessary to give a definite answer.
Similar content being viewed by others
Main
Breast-conserving therapy (BCT) is seen as the first choice treatment modality in early-stage breast cancer. Although there is a common understanding that delay in starting the radiation therapy (RT) in BCT may reduce the probability of local control, the optimum time interval between lumpectomy and RT has not been established. Delays of >8–12 weeks after surgery are still linked to increased risk of local recurrence (Huang et al, 2003; Tsoutsou et al, 2009). No phase III studies evaluating the relevance of the time interval from the lumpectomy to the start of RT have been done.
In most observational studies, local control was the main end point. When looking for the optimum time interval, however, we not only have to take account of the probability of developing local recurrent disease, but also the probability of developing distant metastases and the breast cancer-specific and overall survival.
In 2006, we published our study on ‘timing of radiotherapy and survival benefit in breast cancer’ (Jobsen et al, 2006). We noted a significant adverse effect of earlier timing of RT on the probability of developing distant metastases. That study generated the hypothesis that delaying the start of RT might have a beneficial effect on survival.
We are aware that at present most women get adjuvant systemic therapy, because of extensiveness of the indications. Irrespective of adjuvant therapy, the question remains when to start the RT after lumpectomy. To get an insight in the timing between two treatments, would best be solved by comparing those two treatments without the bias of other treatments.
In this study, we investigated the issue of timing of RT after lumpectomy in a better-defined, more homogeneous cohort. We will try to refine the hypothesis ‘a longer time interval before starting RT after lumpectomy results in an improved distant metastasis-free survival (DMFS) and survival’.
Patients and methods
We used information from our prospective longitudinal cohort of patients diagnosed with invasive breast cancer in the Twente-Achterhoek region between 1983 and 2008 and treated with BCT. A total of 3585 BCT were registered with invasive breast cancer. All patient data, including demographics, pathology, staging information, treatment, and outcome were recorded and updated regularly. Patients were classified according to the TNM-classification, seventh edition. An extensive description of this cohort has been described elsewhere (Jobsen et al, 2006).
We defined bilateral breast cancer (BBC) as cancer diagnosed in both breasts simultaneously or within a period of 3 months of diagnosis of the first tumour. Metachroneous contra lateral breast cancer (CBC) was defined as breast cancer occurring in the contra lateral breast >3 months after the diagnosis of the tumour in the first breast affected.
The cutoff date for analysis of this study was March 2012.
Selection of patients
In an attempt to reduce any bias in prognostic factors and different adjuvant treatments, we created a homogenous group with respect to treatment and nodal status. Only node-negative patients were selected, leaving 2523 BCT.
All patients had their breast RT followed by an external boost. Patients without a boost, those having a peroperative iridium-implant, or those treated according to a phase III trial, and patients with BBC were excluded, leaving 2283 BCT in 2223 women.
Subsequently 390 patients receiving any form of adjuvant systemic therapy were identified.
During the study period guidelines for administering adjuvant systemic therapy in breast cancer have changed. In the early years, the number of tumour-positive axillary lymph nodes was the only indication. Later on not only positive lymph nodes but also large tumour size, high malignancy grade, and young age were of importance. Also the type of chemotherapy and hormone therapy, alone or in combination changed. Hence, over the period of the study, tumour characteristics as well as patient’s characteristics of our cohort became more favourable. Between 1983 and 1990 0.8% of the 2283 node-negative BCT received adjuvant systemic therapy, 2.1% between 1991 and 1996, 26.2% between 1997 and 2002, and 33.0% between 2003 and 2008 (P<0.001).
Having a hormone-positive status was and is no standard indication for adjuvant hormone treatment.
Before 1996, malignancy grading was not routinely reported. Therefore, in order to obtain a homogenous cohort containing optimal comparable subgroups, we restricted the analyses to the inclusion period from 1996 through 2008, all patients are node-negative and without any adjuvant systemic therapy, resulting in 1114 BCT’s in 1075 women. The difference between BCT and women is due to those with CBC treated with BCT.
Treatment
Breast-conserving therapy is defined as lumpectomy followed by irradiation of the whole breast with a boost to the primary tumour area. The lumpectomy was accompanied by axillary clearance of levels I–III and since 2000 sentinel node procedures were standard of care. The radiotherapy regimen consisted of 50 Gy to the whole breast, delivered in 2 Gy fractions five times a week by a tangential field technique. This was followed by a boost of 14 Gy to the primary tumour bed, in 2 Gy fractions five times a week, using either external photon or electron beam therapy. The boost dose was the same in all patients, regardless of margin status.
Definition of timing
During the whole period of this cohort, no guidelines or protocols with respect to the start of the RT were used in treating patients after their lumpectomy. After referral of the patients to our department by the surgeon they were scheduled for treatment. Reasons for the delay in starting RT were related to the variability of time intervals between lumpectomy and axillary dissection, for example, because of a re-excision after lumpectomy, postoperative wound-healing complications, delay in referral to our department, and a waiting list for starting the irradiation. No selection in starting RT was made on any known prognostic factor. Lumpectomy–axillary dissection delay was used as a variable in the analysis.
Timing of RT was defined by the number of days from lumpectomy till start of irradiation. The time-span ranged from 12 to 197 days after lumpectomy. To exclude the extreme time intervals (n=7), we restricted the maximum time till 16 weeks (112 days) after lumpectomy.
This resulted in 1107 BCT with 1070 women for analysis. Owing to the total number, patients were categorised into three groups with respect to timing of RT, according to tertiles: <45 days, 45–56 days, and 57–112 days.
Statistical methods
Time to recurrence and length of follow-up were calculated from the start of BCT. To test between-group differences for categorical data χ2 tests were used, and these analyses with regard to local recurrences were performed in relation to the number of BCT. The local recurrence-free survival (LRFS) is defined as survival without local recurrence.
Survival statistics were performed in relation to the number of patients and calculated by the method of Kaplan and Meier. The disease-specific survival (DSS), corrected for intercurrent death, was also calculated in relation to the number of patients. This means that patients who died of other causes were censored at the date of death. The DMFS is defined as survival without distant metastasis in patients.
For comparison of survival distributions the log-rank test was used.
The Cox proportional hazards model was used to test for the independent effect of timing of RT after adjusting for known prognostic factors and hazard ratios (HRs) estimated with 95% confidence limits are presented. A test for trend across the three ordered tertiles was performed. The tertile with the shortest time interval, <45 days, was the referent group.
All analyses were performed using STATA (Stata/SE 12.1 for Windows; Stata Press, College Station, TX, USA; Stata Corporation, 2011).
Results
The median number of days from lumpectomy till start of irradiation was 50 (interquartile range 41.5–62 days). The follow-up ranged from 5 to 191 months with a median of 94 months.
The tumour and patients characteristics according to the tertiles are shown in Table 1. The tertiles showed a significant difference for age, histology, tumour size, re-excision, and difference in time between lumpectomy–axilla dissection.
The median times between lumpectomy and RT for the 1e, 2e, and 3e tertile were 38, 50, and 69 days, respectively.
Local control
During the study period, 49 (4.4%) ipsilateral recurrences were seen. The LRFS showed no relationship with the timing of RT. The 15-year LRFS were 89.3% for the first tertile, 83.0% for the second (HR 1.4; 95% CI 0.7–2.6; P=0.360), and 46.4% for the third (HR 0.7; 95% CI 0.3–1.6; P=0.416).
Corrected for the significant different patients characteristics as age, re-excision, histology, tumour size, and difference in time between lumpectomy–axilla dissection, timing of RT (Table 1) showed a HR of 2.3 (95% CI 0.6–8.5; P=0.205) for the second tertile, and a HR of 1.2 (95% CI 0.3–6.4; P=0.761) for the third tertile compared with the first tertile.
Distant metastasis
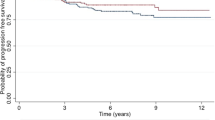
The DMFS rates after 15 years in relation to the timing of RT were 79.8% for the first tertile, 88.5% for the second (HR 0.6; 95% CI 0.4–1.1; P=0.102), and 80% for the third tertile (HR 0.7; 95% CI 0.4–1.1; P=0.120), respectively (Figure 1).
Corrected for age, re-excision, histology, tumour size, and difference in time between lumpectomy–axilla dissection, timing of RT (Table 1) showed a HR of 0.5 (95% CI 0.2–1.1; P=0.116) for the second tertile, and a HR of 0.3 (95% CI 0.1–0.8; P=0.017) for the third tertile compared with the first tertile (Figure 2).
The smoothed hazard HRs estimates for distant metastases according to the three tertiles are shown in Figure 3. The corrected HRs estimates show a clear difference in HRs between the first tertile and the other two, in particular after about 108 months with a steep increase in the HR (Figure 4).
Disease-specific survival
The DSS after 15 years in relation to the timing of RT was 85.0% for the first tertile, 88.6% for the second (HR 0.7; 95% CI 0.4–1.4; P=0.346), and 93.1% for the third tertile (HR 0.6; 95% CI 0.3–1.1; P=0.107), respectively (Figure 5).
Corrected for age, re-excision, histology, tumour size, and difference in time between lumpectomy–axilla dissection timing of RT showed a HR of 0.3 (95% CI 0.1–1.1; P=0.077) for the second tertile, and a HR of 0.2 (95% CI 0.04–0.7; P=0.012) for the third tertile compared with the first tertile.
Discussion
The results of this cohort study further refine the hypothesis that timing of RT in BCT might have an impact on outcome.
By creating a cohort of patients treated only with surgery and RT in BCT, we were able to avoid any bias from other treatments modalities to a great extent. For lymph node-negative breast cancer patients, we noted that an early start of RT might have an adverse effect on the rate of DMFS and DSS. We also showed that an interval time of ⩽112 days had no negative impact on local control.
In forming this cohort, we have tried to avoid any bias, mainly confounding by indication, by including only node-negative cases treated with lumpectomy and RT. All patients were treated according the same radiotherapy regimen in the same institute. All cases received whole breast irradiation followed by a boost to the primary tumour region. However, as with all observational studies it is impossible to exclude the possibility that by chance or subconscious selection, bias could account for the observed results. In this case important differences in age, histological type, timing of surgery of the axilla, and re-excision rates exist. Results were corrected for these items. Re-excision is important for two reasons (a) it inevitably delays the start of RT and (b) in its own right it might be a prognostic factor as tumours with diffuse infiltrating margins are hard to clear at first attempt and might be a surrogate marker for a high-grade tumour and recurrent disease. This might contribute to confounding by indication, meaning that those with the worst prognosis are scheduled for radiation as fast as possible.
We also noticed a difference in re-excisions with a higher rate in the second and third tertiles. The difference can be explained by the adaption of our protocols for re-excision after our paper in 2003 (Jobsen et al, 2003).
Only with regard to the workout of the radiotherapy, planning and dose distributions significant improvements have been implemented over time. During the time of this study, the standard surgery of the axilla changed. Breast-conserving surgery changed from lumpectomy combined with axilla dissection to lumpectomy followed by sentinel node procedure. Owing to changing surgical protocols timing of the treatment of the axilla was not always at the same time as the lumpectomy, resulting in a difference in time between lumpectomy and axilla. This resulted a longer time between lumpectomy and starting RT for different patients. This was in particularly in the period between 1998 and 2002.
In 2008, Chen et al published a systemic review on the relationship between waiting time for RT and clinical outcome. They also looked at the impact on local control in breast cancer, treated with BCT, and without adjuvant chemotherapy. They included four separate studies and concluded that the relative risk was 1.11. Our study from 2006 was not included although it met all inclusion criteria as mentioned in the paper. Looking at the four included studies, in the large study by Froud et al (2000) with 1962 patients 34.5% of the patients received adjuvant hormonal therapy, which might have influenced outcome. Another study from Whelan et al (1996) was presented only as an abstract. Only the study from Vujovic et al (1998) seems to deal with a homogenous group of 568 patients. In this study, only patients with lymph node-negative cancers treated without adjuvant systemic therapy were included. They did not notice any influence on local control of RT administered till 12 weeks after surgery.
Herbert-Croteau et al (2004) looked at the effect of delay in RT on local control and survival in 1062 patients, node negative, of whom 54% received adjuvant hormonal therapy and 20.4% adjuvant chemotherapy. This makes it hard to look at the impact of timing of RT on outcome in their study. Froud et al (2000) did not find a difference in systemic relapses of RT administered up to 12 weeks after surgery, but again 34.5% patients received adjuvant hormonal therapy. Vujovic et al (1998) did not show a significant difference in disease-free survival between the different surgery–radiotherapy intervals.
In a review article, Tsoutsou et al (2009) looked at the optimal timing for adjuvant RT in breast cancer. Looking at the different studies involved in this review, we notice a diversity of treatments, and patients groups. In our opinion it is not possible to extract an optimal timing of RT in BCT or after mastectomy, with different groups of patients.
Our study supports the suggestion that delaying the start of the RT decreased the distant metastases rate and increased the DSS. The optimum delay has to be established, but seems to be >45 days from the results of this study.
The possible explanations for the effect of timing we found on distant metastasis and survival is intriguing and only scarce data are available in the literature. This study showed a worse DMFS for those having their RT early. It also showed in the hazard estimates a clear difference, not only in HRs, but also in the pattern of the estimates. The uncorrected hazard estimates shows a first peak at about 36–48 months, which is quite similar across all three tertiles, and only differs in the size of the HRs. The second peak differs for the tertiles; in contrast to the second tertile with a decrease followed with a slight increase at about 108 months, the first and third tertile show a steep increase. When we look at the corrected estimates the first tertile is not only different in HRs, but also in pattern, with a clear first and second peak compared with the other two. The same is noted for the DSS.
For the answer we have to look at it from two sides. First, this might be caused by confounding factors as age, and other prognostic factors. With respect to the latter we showed that the probability of confounding by indication have been minimised, but cannot be exclude completely. Another probability would be that starting RT too early has an adverse effect on distant metastases.
In 1991, Von Essen published a review on radiation-induced enhancement of metastasis. They concluded that the preclinical evidence for metastasis enhancement by local tumour irradiation suggests two mechanisms: (1) a radiation-induced growth delay mediated by stromal and/or tumour cell damage permitting a constant rate of escape of tumour cells; (2) vascular damage permits an increased rate of escape of tumour cells. Taking into consideration the damage caused by surgery, the immediate following of RT might enhance this damage. Waiting after the surgery might theoretically enable the enhancement of repair of this damage, and might result in a lesser vascular damage and hence less tumour cells.
Angiogenesis has been shown to be essential for the growth and survival of solid tumours and their metastases (Folkman, 1989). Hartford et al (2000) showed that irradiation of a tumour may enhance angiogenic suppression of a tumour deposit at a distal site.
In contrast, Camphausen et al (2001) showed in their study that RT to a primary tumour, analogous to its removal, is followed by the explosive growth of the previously dormant metastatic cells. They suggested that RT might have a complex effect on angiogenesis at a local site as well as a systemic effect.
In a review on the natural history of breast cancer and the punctuated evolution of conceptual models, to explain its behaviour Baum et al (2005) postulated a new model to explain the natural history of the disease. The new model is based on the concept of tumour dormancy/latency, both in the preclinical phase within the breast and later with the micro metastases that seed in the early phase of the natural history of the disease, once the primary focus has developed its own microvasculature. The latter remain dormant until some signal; perhaps the act of surgery or other adverse life events stimulates them into fast growth. The model suggests that the metastatic development of unperturbed breast cancer is a sequential evolution from a non-proliferative to a proliferative state and from a non-angiogenic to an angiogenic state, with stochastic transitions from one state to the next.
One could argue that the growth of previously dormant metastases by enhanced angiogenesis is triggered by the surgery. This study suggests that radiotherapy might also have a role in this process. Baum et al (2005) suggested that one explanation would be that although the number of metastases that are seeded by the primary tumour would be linearly related to the tumour size and biological aggressiveness, the clinical appearance of metastases is triggered or accelerated only after the primary tumour has been disturbed or removed. The rapid start of the RT might increase possible stimulating factors or reduce inhibiting factors resulting in a longer or enhanced effect of the surgery on the dormant condition.
The above-mentioned theories might explain the differences in HRs at the first peak for the tertiles, but do they also explain the late second steep peak?
What is the relevance of our study? The relevance is that timing of RT in BCT seems to have an impact on distant metastasis and survival. This study suggests that a randomised trial in timing of RT in BCT seems necessary to give a definite answer, only ethical considerations might prevent such a study. If confirmed it might have major implications not only on outcome, but also on treatment strategy. Another possibility is to do large retrospective cohort studies, comparing the timing of these two treatments. Many studies nowadays look at the sequence of therapies in the treatment of breast cancer. In our opinion, it might be more interesting to look for the optimum time of any therapy in relation to another. Having these answers it might be easier and more effective to define an optimal sequence of therapies, in which the patient gets the best result in relation to outcome. For example, we should not lose the positive effect of any adjuvant therapy because of a possible loss of a bad timing of RT.
Change history
05 March 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Baum M, Demicheli R, Hrushesky W, Retsky M (2005) Does surgery unfavourably perturb the ‘natural history’ of early breast cancer by accelerating the appearance of distant metastases? Eur J Cancer 41: 508–515
Camphausen K, Moses MA, Beecken WD, Khan KM, Folkman J, O’Reilly MS (2001) Radiation therapy to a primary accelerates metastatic growth in mice. Cancer Res 61: 2207–2211
Chen Z, King W, Pearcy R, Kerba M, Mackillop WJ (2008) The relationship between waiting time for radiotherapy and clinical outcome: a systemic review of the literature. Radioth Oncol 87: 3–16
Folkman J (1989) What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 82: 4–6
Froud PJ, Mates D, Jackson JSH, Phillips N, Andersen S, Jackson SM, Bryce CJ, Olivotto IA (2000) Effect of time interval between breast-conserving surgery and radiation therapy on ipsilateral breast recurrence. Int J Radiat Oncol Biol Phys 46: 363–372
Hartford AC, Gohongi T, Fukumura D, Jain RK (2000) Irradiation of a primary tumor, unlike surgical removal, enhances angiogenesis suppression at a distal site: potential role of host-tumor interaction. Cancer Res 60: 2128–2131
Herbert-Croteau N, Freeman CR, Latreille J, Rivard M, Brisson J (2004) A population-based study of the impact of delaying radiotherapy after conservative surgery for breast cancer. Breast Cancer Res Treat 88: 187–196
Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ (2003) Does delay in starting treatment affect the outcomes of radiotherapy? A systemic review. J Clin Oncol 21: 555–563
Jobsen JJ, van der Palen J, Ong F, Meerwaldt JH (2003) The value of a positive margin for invasive carcinoma in breast conservative treatment in relation to local recurrence is limited to young women only. Int J Radiat Oncol Biol Phys 57: 724–731
Jobsen JJ, van der Palen J, Ong F, Meerwaldt JH (2006) Timing of radiotherapy and survival benefit in breast cancer. Breast Cancer Res Treat 99: 289–294
Tsoutsou PG, Koukourakis MI, Azria D, Belkacemi Y (2009) Optimal timing of radiation therapy in breast cancer. A comprehensive review and perspectives. Crit Rev Oncol Hematol 71: 102–116
Von Essen CF (1991) Radiation enhancement of metastasis: a review. Clin Expl Metastasis 9: 77–104
Vujovic O, Perera F, Dar AR, Stitt L, Yu E, Voruganti SM, Truong PT (1998) Does delay in breast irradiation following conservative breast surgery in node negative breast cancer patients have an impact on risk of recurrence? Int J Radiat Oncol Biol Phys 40: 869–874
Whelan TJ, Clark RM, Levine MN, Willan A, McCulloch P, Lipa M, Wilkinson RH (1996) The effect of delay in initiating radiotherapy post-lumpectomy on local breast recurrence. Int J Radiat Oncol Biol Phys 36: 280
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Jobsen, J., van der Palen, J., Baum, M. et al. Timing of radiotherapy in breast-conserving therapy: a large prospective cohort study of node-negative breast cancer patients without adjuvant systemic therapy. Br J Cancer 108, 820–825 (2013). https://doi.org/10.1038/bjc.2013.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2013.36
Keywords
This article is cited by
-
Clinical relevance of the timing of radiotherapy after breast-conserving surgery
Strahlentherapie und Onkologie (2022)
-
Early intervention with pulse dye and CO2 ablative fractional lasers to improve cutaneous scarring post-lumpectomy: a randomized controlled trial on the impact of intervention on final cosmesis
Lasers in Medical Science (2019)
-
The influence of timing of radiation therapy following breast-conserving surgery on 10-year disease-free survival
British Journal of Cancer (2017)
-
Waiting time for radiation therapy after breast-conserving surgery in early breast cancer: a retrospective analysis of local relapse and distant metastases in 615 patients
European Journal of Medical Research (2016)
-
Potential clinical predictors of outcome after postoperative radiotherapy of non-small cell lung cancer
Strahlentherapie und Onkologie (2014)