Abstract
Background:
The extent to which experiencing a stressful life event influences breast cancer prognosis remains unknown, as the findings of the few previous epidemiological studies are inconsistent. This large population-based study examines the association between a common major life event, loss of a partner and breast cancer recurrence and all-cause mortality.
Methods:
N=21 213 women diagnosed with a first primary breast cancer 1994–2006, who had a cohabiting partner in the 4 years before their breast cancer diagnosis, were followed for death and recurrence in population-based registers and clinical databases. Information on education, disposable income, comorbidity and prognostic risk factors were included in Cox regression analyses.
Results:
Women who had lost a partner either before diagnosis or in subsequent years were not at significantly higher risk of recurrence or dying than women who had not lost a partner.
Conclusion:
Our results do not support the concern that experiencing a stressful life event, the loss of a partner, negatively affects prognosis of breast cancer.
Similar content being viewed by others
Main
A stressful life event may affect prognosis of breast cancer directly through stress-induced alterations of the immune and neuroendocrine system and indirectly through changes in health behaviour, such as physical activity, consumption of alcohol, compliance to therapy and coping with disease. In all, six previous studies have addressed the association between stressful life events and breast cancer prognosis: two of which found a significantly increased risk for recurrence (Ramirez et al, 1989; Palesh et al, 2007), two studies found no association (Hislop et al, 1987; Maunsell et al, 2001), whereas two studies found, contrary to what was expected, a significantly lower risk of recurrence (Barraclough et al, 1992; Graham et al, 2002). These inconsistent findings may be due to the use of different measures of exposure and methodological weaknesses including self-reported measure of exposure, small samples (N=94–665) and selection or recall bias (refer to web appendix: Supplementary Table A1). In this large population-based study, of more than 20 000 breast cancer cases, we use objective information from population-based registers and clinical databases to examine the association between a single life event stressor, loss of a partner and breast cancer prognosis. Loss of a partner is a common and also very stressful life event, implying considerable changes to everyday life (Holmes and Rahe, 1967).
Materials and methods
Linkage of registry data
Information on sex, date of birth, current and historical addresses, emigration, disappearance and death with date of these incidences were obtained from the Central Population Register in which all Danish residents since 1968 have been registered with a personal identification number allowing linkage of information between national registers (Pedersen et al, 2006).
Breast cancer
We obtained information on date of breast cancer diagnosis (defined as date of primary surgery), date of recurrence (which is reported up to 10 years after diagnosis), tumour size (in mm), number of tumour-positive lymph nodes, malignancy grade, hormone receptor status and menopausal status, from Danish Breast Cancer Cooperative Group, which contain information on nearly 95% of all breast cancer cases in Denmark since 1977 (Møller et al, 2008).
Other cancers
Information on first primary cancer, excluding nonmelanoma skin cancer, was obtained from the Danish Cancer Registry, which since 1943 have registered all cases of cancer (ICD-7) in Denmark (Storm et al, 1997).
Stressful life event
A stressful life event was defined as the death of a cohabiting partner either in the 4 years before breast cancer diagnosis or in subsequent years. ‘Cohabitation’ was defined as two persons of the opposite sex over the age of 16, with a maximum age difference of 15 years, living at the same address with no other adults in the residence.
Socioeconomic status and comorbidity
Information on educational level and disposable income (categorised in Table 1) was obtained 2 years before breast cancer diagnosis from the population-based Integrated Database for Labour Market Research in Statistics Denmark with data on sociodemographic factors in Denmark since 1980 (Thygesen, 1995).
Information on comorbidity categorised according to the Charlson comorbidity index (scores 0, 1 and ⩾2), excluding cancers (Charlson et al, 1987) was obtained from the Danish National Patient Register with information on all somatic diseases leading to hospitalisation since 1977, and from 1995 also information on all outpatient visits (Andersen et al, 1999).
Analysed cohort
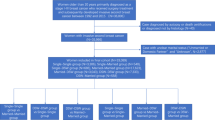
To attain accurate information from the included registers our base population is restricted to all 3.4 million Danish residents born between 1925 and 1973 who resided in Denmark 1994–2006 and entered the cohort at age 30 (for more details Dalton et al, 2008). From this population we identified 22 366 women diagnosed with breast cancer between 1 January 1994 and 31 December 2006 with no previous history of cancer (except nonmelanoma skin cancer), who resided in Denmark 2 years before diagnosis, and had a cohabiting partner up to 4 years before their breast cancer diagnosis. We excluded 1153 women with missing values on one or more covariates or who had resided in Denmark for less than 2 years. In all, 21 213 eligible women were followed for recurrence and death.
Statistical analyses
We used Cox regression to assess hazard ratios (HRs) with 95% confidence intervals (CIs) for all-cause mortality and breast cancer recurrence, respectively, according to the vital status of the partner. For all-cause mortality follow-up time was counted from the date of diagnosis until death, emigration or 31 December 2010, whichever came first. For breast cancer recurrence follow-up time was counted from the date of diagnosis until death, emigration, 10 years of follow-up or 31 December 2006, whichever came first. The exposure, death of partner (after diagnosis), was included as a time-dependent variable, so that person–time before the death of the partner was counted as unexposed, whereas person–time after the date of death of the partner was counted as exposed. In all analyses, time since breast cancer diagnosis was used as the underlying time scale, and baseline hazards were allowed to vary across age at breast cancer diagnosis in 1-year intervals. The HRs were first adjusted for educational level and income, both considered as potential confounders. Subsequently, we adjusted for comorbidity, period of diagnosis, tumour size, number of positive lymph nodes, receptor status and malignancy grade (I–IV), as these factors are strongly associated with outcome, however, not obviously associated with the exposure. We estimated HRs in the intervals [0–1],]1–2],]3–4] years for exposure before diagnosis and for latencies of: [0–2],] 2–5],]5–17] years for exposure after diagnosis. We investigated whether a change in cohabitation status influenced the estimated association, by censoring at 1 January in the year in which the cohabitation status changed. Further, we estimated the association for women diagnosed with hormone-receptor-positive breast cancer and post-menopausal women only.
Results
In the analysis of the association between loss of partner and all-cause mortality 172 773 person–years of follow-up were accrued, with a median follow-up of 7.7 years (ranging 0–17). During follow-up, 5660 women died, 762 lost their partner in the 4 years before their breast cancer diagnosis and 2259 lost their partner during follow-up. As expected, women who lost their partner were older and had a lower education and income compared with those who did not (Table 1). After adjustment for education and income as well as period of diagnosis, comorbidity and severity of breast cancer, women who had lost a partner were not at a significantly higher risk for recurrence or all-cause death from that of women who did not lose a partner, no matter if the event happened in the 4 years before diagnosis or in subsequent years (Table 2) or at different latencies (Table 3). We found only minor changes to the estimates when censoring at change in cohabitation status and when measuring the association only among women diagnosed with hormone-receptor-positive breast cancer or post-menopausal women (results not shown).
Discussion
Our results do not support the concern that experiencing a major stressful life event, loss of a partner, negatively affects breast cancer recurrence or all-cause mortality, whether the event occurs in the 4 years before or 0–17 years after diagnosis. Of six previous studies four support this finding (Hislop et al, 1987; Barraclough et al, 1992; Maunsell et al, 2001; Graham et al 2002). Two previous studies found a higher risk of recurrence among women reporting stressful life events, however, both were of retrospective design and the observed estimate may reflect differential recall and reporting of events (Ramirez et al, 1989; Palesh et al, 2007).
We addressed several methodological limitations of the previous studies. First, we included more than 30 times as many cancer patients as the largest study published so far. Second, the use of national registers and databases to identify the study population ensures high representativeness and minimal risk for selection bias. Third, the exposure (death of partner) was measured independently of the participants, eliminating recall bias. Fourth, the register-based cohort design ensures temporality and minimises loss to follow-up and misclassification of outcomes. Finally, we estimated the effect of the death of partner before and after diagnosis on both recurrence and all-cause mortality.
Still, loss of partner is relatively rare among newly diagnosed breast cancer patients, and 73% of the cohort was alive and recurrence free at exit, resulting in small number of events in certain subgroups. The observed estimates of all-cause mortality may represent overestimates of breast cancer-specific mortality. We adjusted, however, the analyses for comorbidity and investigated also recurrence as outcome. Finally, an association may be present among women with poor coping resources or accumulated stressful life events. We were unable to take these into account.
The death of a partner is a common major life event, and our finding of no association with breast cancer recurrence or all-cause mortality may provide reassurance for women confronting breast cancer.
Change history
23 January 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Andersen TF, Madsen M, Joergensen J, Mellemkjoer L, Olsen JH (1999) The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull 46: 263–268
Barraclough J, Pinder P, Cruddas M, Osmond C, Taylor I, Perry M (1992) Life events and breast cancer prognosis. BMJ 302: 1078–1081
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbiduty in longitudinal studies: development and validation. J Chron Dis 40: 373–383
Dalton SO, Steding-Jesen M, Gislum M, Frederiksen K, Engholm G, Schüz J (2008) Social inequality and incidence of and survival from cancer in a population-based study in Denmark, 1994–2003: background, aims, material and methods. Eur J Cancer 44: 1938–1949
Graham J, Ramirez A, Love S, Richards M, Burgess C (2002) Stressful life experiences and risk of recurrence of breast cancer: observational cohort study. BMJ 324: 1420–1423
Hislop TG, Waxler NE, Coldman AJ, Elwood JM, Kan L (1987) The prognostic significance of psychosocial factors in women with breast cancer. J Chronic Dis 40: 729–735
Holmes TH, Rahe RH (1967) The social readjustment rating scale. J Psychosom Res 11: 213–218
Maunsell E, Brisson J, Mondor M, Verreault R, Deschenes L (2001) Stressful life events and survival after breast cancer. Psychosom Med 63: 306–315
Møller S, Jensen MB, Ejlertsen B, Bjerre KD, Larsen M, Hansen HB, Christiansen P, Mouridsen HT, Danish Breast Cancer Cooperative Group (2008) The clinical database and the treatment guidelines of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol 47: 506–524
Palesh O, Butler LD, Koopman C, Giese-Davis J, Carlson R, Spiegel D (2007) Stress history and breast cancer recurrence. J Psychosom Res 63: 233–239
Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB (2006) The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bul 53: 441–449
Ramirez AJ, Craig TK, Watson JP, Fentiman IS, North WR, Rubens RD (1989) Stress and recurrence of breast cancer. BMJ 298: 291–293
Storm HH, Michelsen EV, Clemmensen IH, Pihl K (1997) The Danish Cancer Registry- history, content, quality and use. Dan Med Bull 44: 535–539
Thygesen L (1995) The register-based system of demographic and social statistics in Denmark-an overview. Stat J UN Econ Commun Eur 12: 49–55
Acknowledgements
This work was funded by the Health Insurance Foundation; and the Danish Cancer Society. For their support in data management we thank Visti Birk Larsen, MD, and Marianne Steding-Jessen MSci, Survivorship, Danish Cancer Society Research Center.
Author contributions
All authors have contributed to the conception and design or analysis and interpretation of data and approved the final version of the report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Olsen, M., Bidstrup, P., Frederiksen, K. et al. Loss of partner and breast cancer prognosis — a population-based study, Denmark, 1994–2010. Br J Cancer 106, 1560–1563 (2012). https://doi.org/10.1038/bjc.2012.96
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.96
Keywords
This article is cited by
-
The relation between stressful life events and breast cancer: a systematic review and meta-analysis of cohort studies
Breast Cancer Research and Treatment (2019)


