Abstract
Aim:
Psm2, one of the pyrrolidinoindoline alkaloids isolated from whole Selaginella moellendorffii plants, has shown a potent antiplatelet activity. In this study, we further evaluated the antiplatelet effects of Psm2, and elucidated the underlying mechanisms.
Methods:
Human platelet aggregation in vitro and rat platelet aggregation ex vivo were investigated. Agonist-induced platelet aggregation was measured using a light transmission aggregometer. The antithrombotic effects of Psm2 were evaluated in arteriovenous shunt thrombosis model in rats. To elucidate the mechanisms underlying the antiplatelet activity of Psm2, ELISAs, Western blotting and molecular docking were performed. The bleeding risk of Psm2 administration was assessed in a mouse tail cutting model, and the cytotoxicity of Psm2 was measured with MTT assay in EA.hy926 cells.
Results:
Psm2 dose-dependently inhibited human platelet aggregation induced by ADP, U4619, thrombin and collagen with IC50 values of 0.64, 0.37, 0.35 and 0.87 mg/mL, respectively. Psm2 (1, 3, 10 mg/kg) administered to rats significantly inhibited platelet aggregation ex vivo induced by ADP. Psm2 (1, 3, 10 mg/mL, iv) administered to rats with the A–V shunt dose-dependently decreased the thrombus formation. Psm2 inhibited platelet adhesion to fibrinogen and collagen with IC50 values of 84.5 and 96.5 mg/mL, respectively, but did not affect the binding of fibrinogen to GPIIb/IIIa. Furthermore, Psm2 inhibited AktSer473 phosphorylation, but did not affect MAPK signaling and Src kinase activation. Molecular docking showed that Psm2 bound to phosphatidylinositol 3-kinase β (PI3Kβ) with a binding free energy of −13.265 kcal/mol. In addition, Psm2 did not cause toxicity in EA.hy926 cells and produced only slight bleeding in a mouse tail cutting model.
Conclusion:
Psm2 inhibits platelet aggregation and thrombus formation by affecting PI3K/Akt signaling. Psm2 may be a lead compound or drug candidate that could be developed for the prevention or treatment of thrombotic diseases.
Similar content being viewed by others
Introduction
Thrombosis plays a crucial role in the development of some cardiovascular disorders, including acute coronary syndrome, myocardial infarction and pulmonary embolism1. In the process of thrombus formation, platelets adhere to the vascular wall, become activated, release agonists and finally aggregate at the injured vascular site2,3. Therefore, antiplatelet agents, including ticlopidine, aspirin and the glycoprotein IIb–IIIa (GPIIb/IIIa) inhibitor tirofiban, are used for the treatment and prevention of cardiovascular thrombotic diseases4,5. However, these agents may produce hemorrhagic events or upper gastrointestinal bleeding, which limits their clinical application6,7. Recently, PI3K/Akt signaling has been discovered to be a valuable antithrombotic therapy target without a significant effect on primary hemostasis8. At present, more than 50 inhibitors targeting PI3K/Akt signaling are under evaluation in clinical trials of various phases9.
Novel antiplatelet agents can be derived from various sources, including dietary and medicinal plants, which can contain natural compounds that exhibit antiplatelet/thrombotic properties or control cardiovascular diseases10. In recent decades, many constituents with biological activities have been isolated from Chinese herbs that have been used as remedies for thousands of years11.
The family Selaginellaceae Willk. includes the single genus Selaginella Beauv. Selaginella is a genus with a distribution that is almost worldwide. It includes approximately 700 species, with 72 species in China, of which more than 20 species are used in traditional Chinese medicine12,13. Several Selaginella species, including S delicatula (Desv ex Poir) Alston, S moellendorffii Hieron, S nipponica Franch & Sav, S sanguinolenta (L) Spring, S stauntoniana Spring, and S tamariscina (P Beauv.) Spring are used to promote blood circulation (Huoxue in Chinese)12. However, compounds derived from these species have not been described to have therapeutic effects in cardiovascular diseases.
Previously, we isolated and identified eight pyrrolidinoindoline alkaloids from the S moellendorffii plant14. After screening for platelet aggregation activity, we found that one compound, Psm2 (Figure 1), exhibited potent antiplatelet activity. In the present study, we studied the effects of Psm2 on platelet aggregation and thrombus formation and the underlying mechanism.
Chemical structure of Psm2.
Materials and methods
Reagents and chemicals
Psm2 (HPLC grade with ≥95%) was obtained from Dr Yue-hu WANG's laboratory (Kunming Institute of Botany, Chinese Academy of Sciences). Adenosine diphosphate (ADP), thrombin, U46619, heparin, human fibrinogen, anti-mouse IgG-alkaline phosphatase antibody, p-nitrophenyl phosphate substrate, prostaglandin E1 (PGE1) and aspirin were purchased from Sigma (St Louis, MO, USA). Collagen (type I) was purchased from Hyphen-Biomed (Neuville-sur-Oise, France). Human platelet GPIIb/IIIa was purchased from Enzyme Research Laboratories (South Bend, IN, USA) and mouse anti-human integrin β3 antibody was purchased from Millipore (Billerica, MA, USA). Anti-Akt, anti-phospho-Akt, anti-p38, anti-phospho-p38, anti-Src, and anti-phospho-Src antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). The PI3Kβ inhibitor TGX-221 was purchased from Cayman Chemical (Ann Arbor, MI, USA). The EA.hy926 cells were obtained from the Cell Bank of Shanghai Institute of Biochemistry & Cell Biology (Shanghai, China), and all other chemicals used in this research were of analytical grade.
Animals and human samples
The Institute of Cancer Research (ICR) mice (n=50, 18–22 g) and Sprague-Dawley (SD) rats (n=122, 180–250 g) were purchased from the Qinglongshan Animal Center (Nanjing, Jiangsu, China). All animals were maintained under specific pathogen-free conditions at a controlled temperature of 22–24 °C and a relative humidity of 55%–65% with a 12 h dark/light cycle and given free access to food and water. All experiments were carried out in accordance with the guidelines and the regulations of the Ethical Committee of the China Pharmaceutical University. All the animal protocols were approved by the Animal Care and Use Committee at China Pharmaceutical University. Human venous blood was obtained from healthy donors in accordance with the Declaration of Helsinki and permission from the Ethical Committee of the China Pharmaceutical University. Written informed consent was obtained from all participants.
Preparation of platelet-rich plasma and platelet-poor plasma
Rat platelet-rich plasma (PRP) and platelet-poor plasma (PPP) were prepared as previously described15. Briefly, blood samples were drawn from drug-free rats without stasis into a 3.8% sodium citrate solution (9:1, v/v). The blood samples were collected and centrifuged for 15 min at 150×g to obtain PRP or for 10 min at 800×g to obtain PPP. Human platelet-rich plasma (PRP) was obtained from the Jiangsu Province Blood Center.
Platelet aggregation in vitro
Agonist-induced platelet aggregation was measured using a platelet aggregometer (LBY-NJ4, Pulisheng Instrument Co Ltd, Beijing, China) according to a method previously reported16,17,18. Briefly, after calibration of the platelet aggregometer, human PRP was incubated with Psm2 at concentrations of 0.3, 0.5, 1.0, 1.5, and 2.0 mg/mL or vehicle for 5 min at 37 °C without stirring before the addition of ADP, thrombin, U46619 or collagen at different concentrations. Then, the agonist was added with stirring to observe the percent aggregation, and the data are expressed as the percentage of maximal aggregation.
Rat platelet aggregation ex vivo
Rat platelet aggregation ex vivo was measured according to a method previously reported19. Forty SD rats were randomly divided into 5 groups containing 8 rats in each group (50% males). The rats were treated with Psm2 (1, 3, and 10 mg/kg), aspirin (50 mg/kg) or vehicle. Thirty minutes after the treatment was administered, blood samples were obtained from the rats and were anticoagulated with 3.8% sodium citrate solution (9:1, v/v). Then, 290 μL of rat PRP were added into a cuvette and incubated at 37 °C for 5 min before the addition of 10 μL of ADP (10 μmol/L) with stirring. Platelet aggregation was estimated and the data are expressed as described above.
Thrombosis in an arteriovenous shunt in rats
The antithrombotic activity of Psm2 was determined by measuring the thrombus formation in an arteriovenous shunt tube with a modified method17,20,21. Forty SD rats were randomly divided into 5 groups containing 8 rats in each group (50% male). The rats were treated with Psm2 (1, 3 and 10 mg/kg, iv), aspirin (50 mg/kg, iv) or vehicle. Thirty minutes after the treatment was administered, the rats were anesthetized with chloraldurat (10 %, 300 mg/kg, ip) and an A–V shunt tube was inserted between the right carotid artery and the left jugular vein. The wet weight of thrombus was determined by subtracting the weight of the thread soaked with blood from the total weight immediately, and the dry weight was measured 6 h later at room temperature by subtracting the weight of the dry 10-cm thread.
Cytotoxicity assay
To determine the cytotoxicity of Psm2 in vitro, an MTT colorimetric assay was performed with EA.hy926 cells as previously described22. The survival rate of cells was calculated using the following formula: survival rate (%)=(absorbance of treated group–absorbance of blank control)/(absorbance of negative control–absorbance of blank control)×100%23.
Tail bleeding assay in mice
To evaluate the bleeding risk of Psm2, a modified tail cutting method was used24,25. The mice were grouped, and vehicle, Psm2 (3, 10, and 30 mg/kg, iv) or aspirin (50 mg/kg, iv) was administered.
Preparation and labeling of the washed rat platelets
Washed rat platelets were prepared and labeled as described previously10,26. The rats were bled from the carotid artery under chloraldurat anesthesia (300 mg/kg, ip). Blood samples were collected, and PRP was obtained; PRP was then centrifuged at 500×g for 8 min to prepare platelet pellets. The platelet pellets were resuspended in HBMT (4.0 g of NaCl, 2.4 g of HEPES, 0.1 g of KCl, 0.2 g of NaH2PO4·H2O, 0.5 g of BSA, 0.5 g of glucose, and 0.2 g of MgCl2·6H2O in 500 mL of H2O; pH 7.4) containing 1 μg/mL PGE1 (HBMT/PGE1) and then centrifuged for 5 min at 500×g. The platelet pellets were subsequently resuspended in HBMT containing 14 μmol/L calcein-AM and incubated for 30 min at 22 °C in the dark. The labeled platelets were washed with HBMT/PGE1 and resuspended in HBMT containing 2 mmol/L CaCl2 (HBMT/CaCl2) at a platelet concentration of 5.0×108/mL.
Platelet adhesion assay
Platelet adhesion to fibrinogen and collagen was studied as described previously26,27,28. Briefly, 100 μL of human fibrinogen [100 μg/mL in 100 mmol/L NaCl, 50 mmol/L Tris, pH 7.4 (Tris/saline)] or 100 μL of Type 1 collagen (50 μg/mL) was added to FluoroNunc 96-Well plates (Nunc, Albertslund, Denmark). After incubating at 4 °C overnight, the plates were washed 3 times with 200 μL of Tris/saline, and then the wells were blocked with 200 μL of HBMT containing 2% BSA for at least 1 h. After 3 washes, 50 μL of Psm2 at different concentrations and 50 μL of calcein-labeled platelets (5.0×108/mL) were added to each test well and incubated at room temperature in the dark. After 2 h, the wells were washed 4 times with HBMT/CaCl2. PI3Kβ inhibitor TGX-221 served as the positive control. Finally, the fluorescence intensity of the Calcein-labeled platelets was measured with a filter set of 490 nm EX/515 nm EM using an automated microtiter plate reader (Tecan, Infinite® 200 PRO).
GPIIb/IIIa binding assay
GPIIb/IIIa binding was measured in 96-well polystyrene micro-plates according to a previously described method29,30. Psm2 (0.3, 0.1, or 0.03 mg/mL), tirofiban (1 μmol/L) or vehicle was added to the wells. The absorbance of the wells were read at 405 nm using a microplate reader (ELx800, BioTek, USA).
Preparation of gel-filtered human platelets
Gel-filtered human platelets were prepared as described by Yi et al and Prevost et al31,32 with some modifications. Human blood was collected from antecubital veins of healthy volunteers who were not taking medication during the 2 weeks preceding venipuncture. The blood was drawn without stasis into siliconized vacutainers containing a 1:5 volume of ACD (8.38 g of sodium citrate, 7.35 g of citric acid and 9.01 g of dextrose in 500 mL of H2O; pH 4.4). PRP was prepared by centrifugation at 150×g for 20 min at room temperature. PRP was applied to the column that was packed with SepharoseTM 2B beads in Tris-HCl buffer, and the platelets were eluted using Ca2+-free HEPES-modified Tyrode buffer (HBMT) in a series of 1.5-mL tubes. The platelets collected in each tube were counted and adjusted to 2.5×108/mL using HBMT.
Immunoblot assay
Immunoblot assays were performed as described previously10,32. Aliquots of gel-filtered platelets (270 μL, 2.5×108/mL) were preincubated with 20 μL of vehicle, TGX-221 or Psm2 for 10 min and were then stimulated by 10 μL of agonist (ADP, U46619, collagen or thrombin) for 5 min under stirring at 37 °C. The reaction was stopped by the addition of RIPA buffer (1% Triton X-100, 1% deoxycholate, 0.1% SDS, 10 mmol/L Tris, 150 mmol/L NaCl containing protease inhibitors and phosphatase inhibitors). After heating at 100 °C for 5 min, the samples were stored at -20 °C. The proteins were separated by 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE). They were then transferred to a poly vinylidene difluoride (PVDF) membrane and subjected to detection via incubation with the corresponding antibodies. After incubation with the corresponding secondary antibodies for 2 h at room temperature, densitometric band scanning was performed using a Tanon infrared imaging system.
Molecular docking
Molecular Operating Environment (MOE, version 2009.10) software was used to predict the binding mode of Psm2 to PI3K. The X-ray crystallographic structures of different isoforms of PI3K obtained from the RSCB Protein Data Bank were applied to study the protein-ligand docking. The co-crystallized ligand in the crystalline complex was removed prior to docking and replaced by Psm2. The docking result was evaluated using Molecular Mechanics Generalized Born/Volume Integral (MM/GBVI) binding free energy, and the docking energy of Psm2 was finally compared with that of the co-crystallized ligand by docking its co-crystallized ligand to the protein receptor33.
Statistical analysis
All analyses were performed using GraphPad Software 6.0 (Graph Pad, USA). The data are presented as the mean±SEM/SD. The statistical significance of differences was assessed by one-way analysis of variance (ANOVA), followed by the t-test or the Student-Newman-Keuls test (SNK) when appropriate. The IC50 values and 95% confidence intervals were determined by nonlinear regression analysis using a normalized response-variable slope of individual experiments. P<0.05 was considered statistically significant. Quantitation of normalized optical density was performed with Image J software.
Results
The effects of Psm2 on platelet aggregation both in vitro and ex vivo
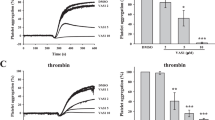
Platelet aggregation induced by various agonists was measured using a platelet aggregation analyzer. As shown in Figure 2A, Psm2 significantly inhibited the aggregation of human platelets induced by ADP (0.91 μmol/L), U46619 (0.83 μmol/L) collagen (0.67 μg/mL), or thrombin (0.26 U/mL) with IC50 values of 0.64 mg/mL (95% CI, 0.54–0.76 mg/mL), 0.37 mg/mL (95% CI, 0.33–0.40 mg/mL), 0.35 mg/mL (95% CI, 0.32–0.39 mg/mL), and 0.87 mg/mL (95% CI, 0.78–0.98 mg/mL), respectively. In addition, Psm2 decreased ADP (10 μmol/L)-induced rat platelet aggregation ex vivo in a dose-dependent manner.
The antiplatelet activities and antithrombotic effects of Psm2. (A) Human PRP was preincubated for 5 min with Psm2 (0.3, 0.5, 1.0, 1.5, and 2.0 mg/mL) or vehicle. Platelet aggregation was initiated with ADP (0.91 μmol/L), U46619 (0.83 μmol/L), thrombin (0.26 U/mL) or collagen (0.67 μg/mL). (B) The rats were treated with Psm2 (1, 3, and 10 mg/kg), aspirin (50 mg/kg) or vehicle. Thirty-minute blood samples were drawn, and PRP was prepared, and then 290 μL of PRP was incubated at 37 °C for 5 min. After the addition of 10 μL ADP (10 μmol/L), the platelet aggregation was tested. (C) The effects of Psm2 on arterial thrombus formation in rats. The tested samples or vehicle was administered intravenously 30 min before the thrombogenic challenge. Mean±SEM. n=8. *P<0.05, **P<0.01 vs the control group.
The antithrombotic activity of Psm2 in vivo
An arterial thrombus formation model was used to evaluate the antithrombotic effects of Psm2 in vivo. As shown in Figure 2C, Psm2 administration (1, 3, and 10 mg/kg) led to a dose-dependent reduction in both the wet thrombus weight (by 26.5%±5.3%, 38.4%±5.6%, and 67.5%±6.1%) and the dry thrombus weight (by 25.9%±3.1%, 36.9%±2.8%, and 49.9%±2.9%) in the arteriovenous shunt thrombosis model. At a dose of 50 mg/kg, aspirin inhibited wet and dry thrombus growth by 62.0%±5.9% and 48.3%±5.2%, respectively, which was comparable to the inhibition mediated by Psm2 (10 mg/kg).
The side effects of Psm2
To examine the side effects of Psm2 administration, Psm2 cytotoxicity and mouse tail bleeding were examined. As shown in Figure 3A, Psm2 had no significant effects on the growth of EA.hy926 cells, with a viability of over 90%, even at a concentration of 6 mg/mL, which was 20-fold higher than the concentration in assays showing an antithrombotic effect of Psm2, indicating that Psm2 had no cytotoxicity. In addition, Psm2 slightly prolonged the bleeding time (Figure 3B). The bleeding times in mice that received Psm2 at doses of 3 and 10 mg/kg were 10.3±2.9 min and 13.7±4.2 min, respectively, which was not markedly different from that of the vehicle-treated group (10.3±3.6 min) and was negligible when compared to that of the aspirin treated-group (18.1±5.7 min). Increasing the dose of Psm2 to 30 mg/kg (3-fold higher than the dose causing similar antithrombotic activity to 50 mg/kg aspirin), the bleeding time increased slightly (15.9±5.4 min, P<0.05), but the bleeding time was less than that of the aspirin treated-group. These results indicated that Psm2 had no cytotoxicity and had a lower bleeding risk than aspirin.
The side effects of Psm2. (A) The cytotoxicity of Psm2. An MTT assay was used to detect the cytotoxicity of Psm2 on EA.hy926 cells. After treatment with Psm2 at different concentrations for 24 h, the survival rate of EA.hy926 cells was assessed. (B) The effect of Psm2 on bleeding time. The bleeding time was recorded for 30 min after bleeding stopped, and the sum of bleeding times was used if bleeding on/off cycles occurred. The data are presented as the mean±SEM. n=10/group. *P<0.05, **P<0.01 vs the control group.
The effects of Psm2 on platelet adhesion and GPIIb/IIIa binding
To explore the possible antiplatelet mechanism of Psm2, a GPIIb/IIIa binding assay and a platelet adhesion assay were performed. Similar to the PI3Kβ inhibitor TGX-221, Psm2 markedly inhibited platelet adhesion to fibrinogen and collagen with IC50 values of 84.5 μg/mL (95% CI, 7.3–12.0 μg/mL) and 96.5 μg/mL (95% CI, 7.0–9.9 μg/mL), respectively. When the concentration of Psm2 was increased to 0.3 mg/mL, the adhesion (%) to fibrinogen was 30.7%, and the adhesion (%) to collagen was 37.5% (Figure 4A, 4B). However, Psm2 showed no effects on GPIIb/IIIa binding, even at an increased concentration of 0.3 mg/mL (1 mmol/L), while tirofiban (1 μmol/L) markedly inhibited the binding of GPIIb/IIIa to fibrinogen with an inhibition ratio of 97.4% (Table 1). This suggested that Psm2 inhibited platelet aggregation by influencing the common pathway that activates the GPIIb/IIIa receptor but not by blocking fibrinogen binding to activated GPIIb/IIIa receptors.
The effects of Psm2 on platelet adhesion. Calcein-labeled washed rat platelets (5×108/mL) were preincubated with Psm2 (0.3, 0.1, 0.03, 0.01, or 0.003 mg/mL) or vehicle for at least 1 h at 37 °C and allowed to spread on fibrinogen/collagen-coated plates. The number of adherent platelets relative to control group was measured using an automated microtiter plate reader, which was expressed as adhesion (%). (A) The effects of Psm2 on platelet adhesion to fibrinogen. (B) The effects of Psm2 on platelet adhesion to collagen. The data are presented as the mean±SD. n=3. *P<0.05, **P<0.01 vs the control group.
The effect of Psm2 on platelet intracellular signaling
To investigate the possible intracellular signaling target of Psm2, we monitored three important platelet signaling pathways, including the PI3K pathway (AktSer473 phosphorylation as the activation marker), the MAPK pathway (p38 phosphorylation as the activation marker), and Src kinase activation. Before being challenged with saline, ADP, U46619, thrombin or collagen, the gel-filtered human platelets were preincubated in the presence or absence of Psm2 (0.3 mg/mL, 37 °C, 10 min). Then, the phosphorylation of Src, Akt and p38 was examined. The results of the immunoblot assay showed that each agonist markedly increased the phosphorylation of these three enzymes and that Psm2 had no influence on Src or p38 phosphorylation, but AktSer473 phosphorylation was markedly inhibited by all four of the tested agonists, which indicated that Psm2 affected PI3K signaling in platelets (Figure 5).
The effects of Psm2 on platelet intracellular signaling. Gel-filtered human platelets (2.5×108/mL) were preincubated with Psm2 (0.3 mg/mL) or vehicle, followed by stimulation with ADP (10 μmol/L), U46619 (U) (3 μmol/L), collagen (COL) (2 μg/mL) or thrombin (THR) (1 U/mL) under stirring at 900 r/min in an aggregometer at 37 °C. The platelets were lysed, and the proteins in the lysates were immunoblotted using antibodies specific for the indicated proteins. (A) Src, (B) PI3K/AktSer473, (C) MAPK/p38. n=3. Mean±SEM. **P<0.01 vs the agonist-induced group. NS indicates no significance.
PI3K is a critical transmitter of intracellular signaling during platelet activation and aggregation. To further clarify how Psm2 affected PI3K signaling, the PI3Kβ inhibitor TGX-221 was employed. Psm2 and TGX-221 were found to inhibit AktSer473 phosphorylation similarly, by potently inhibiting ADP-, collagen- and U46619-induced Akt phosphorylation and by slightly inhibiting thrombin-induced AktSer473 phosphorylation. Moreover, when the concentration of agonists increased, the inhibitory effects of Psm2 and TGX-221 decreased, except following ADP stimulation (Figure 6A), which was consistent with the platelet aggregation results. Furthermore, Psm2 had no influence on early (60 s) AktSer473 phosphorylation but inhibited the late (180 s) AktSer473 phosphorylation (Figure 6B).
The effects of Psm2 on PI3K signaling. (A) Gel-filtered platelet suspensions were preincubated with Psm2 (0.3 mg/mL), TGX-221 (10 μmol/L) or vehicle, followed by stimulation with ADP (10 or 20 μmol/L), U46619 (3 or 6 μmol/L), collagen (2 or 4 μg/mL) or thrombin (1 or 2 U/mL). The phosphorylation of Akt was detected by Western blotting. (B) The platelets were treated with Psm2 (0.3 mg/mL) or vehicle and then were stimulated by thrombin (1 U/mL). The phosphorylation of Akt was measured 0, 20, 60, or 180 s after thrombin stimulation. n=3. Mean±SEM. *P<0.05, **P<0.01 vs the agonist-induced group. NS indicates no significance.
Molecular docking
To explore the possible interaction between Psm2 and PI3K, molecular docking of Psm2 with different isoforms of PI3K was performed. We found that Psm2 could combine with the PI3Kβ/GDC-0941 complex (PDB: 2Y3A) but had no interaction with other isoforms of PI3K. The MM/GBVI binding free energy of Psm2 to PI3Kβ/GDC-0941 was −13.265 kcal/mol. As shown in Figure 7, Psm2 fits the pocket that consists of the Asp917, Asp931, Lys776, Lys799, Met773, and Ser775 residues of the non-conserved regions at the binding site in PI3Kβ/GDC-0941, and hydrogen bonds are also formed between Psm2 and two residues (Lys776 and Lys799) of PI3Kβ/GDC-0941. The docking results implied that Psm2 influenced PI3K signaling and inhibited the platelets, possibly via inhibiting PI3Kβ activities.
Molecular docking results of Psm2 in the PI3Kβ active site (PDB code 2Y3A). The green lines represent hydrogen bonds formed by Psm2 with PI3Kβ/GDC-0941, and the gray lines represent the pocket formed by the Asp917, Asp931, Lys776, Lys799, Met773, and Ser775 residues of the non-conserved regions at the binding site in PI3Kβ/GDC-0941.
Discussion
In this study, we found that Psm2 from S moellendorffii inhibited human platelet aggregation induced by various agonists in vitro and rat platelet aggregation induced by ADP, as well as attenuated thrombus formation as evaluated by an arteriovenous shunt thrombosis model. These results support the traditional usage of S moellendorffii Hieron in promoting blood circulation. Mechanistically, Psm2 inhibited platelet adhesion to fibrinogen and collagen without affecting the binding of fibrinogen to GPIIb/IIIa. Psm2 markedly inhibited AktSer473 phosphorylation, an important downstream effector of PI3K but had no influence on MAPK signaling and Src kinase activation. Additionally, the cytotoxicity assay and tail bleeding assay showed that Psm2 had no cytotoxicity in vitro and had a low bleeding risk.
An agent that inhibits the platelet aggregation induced by various agonists is likely to act through a common signaling pathway. The activation of the GPIIb/IIIa receptor is a common final step of platelet aggregation in response to various agonists, after which αIIbβ3 binds to soluble fibrinogen and bridges platelets, leading to platelet aggregation34. Antagonists of the GPIIb/IIIa receptor inhibit platelet aggregation induced by various agonists by preventing fibrinogen from binding to activated GPIIb/IIIa receptors35. Psm2 inhibited ADP-, thrombin-, U46619-, and collagen-induced platelet aggregation and blocked platelet adhesion to immobilized fibrinogen and collagen without affecting the binding of the GPIIb/IIIa receptor to fibrinogen, which suggested that Psm2 affects inside-out platelet signaling.
Similar to the PI3Kβ inhibitor SAA10, Psm2 showed stronger inhibitory activity on ADP-, collagen-, and U46619-induced platelet aggregation than on thrombin-induced platelet aggregation. With increasing concentrations of the agonists, except for ADP, the inhibition of platelet aggregation decreased markedly, suggesting that collagen- and U46619-induced platelet aggregation largely depend on ADP secretion, which has previously been suggested by Huang et al10. These results imply that Psm2 functions as an inhibitor of PI3K in platelets and thereby prevents GPIIb/IIIa receptor activation, leading to the inhibition of platelet aggregation.
The PI3K pathway, the MAPK pathway and Src kinase are three important platelet signaling pathways, which make important contributions to the activation of GPIIb/IIIa receptors10. The immunoblotting results showed that Psm2 potently blocked the phosphorylation of AktSer473 (a downstream effector of PI3K) induced by various agonists but had no effects on the activation of Src kinase and the phosphorylation of p38 (a downstream effector of MAPK pathway), which indicated that Psm2 affected the PI3K pathway. Consistent with the platelet aggregation results, Psm2 inhibited collagen-, ADP- and U46619-induced AktSer473 phosphorylation more potently than thrombin-induced AktSer473 phosphorylation, and the inhibitory effects on AktSer473 phosphorylation decreased when increasing the concentration of the agonists, except for ADP. Moreover, it is known that early Akt phosphorylation induced by thrombin is downstream of phospholipase C (PLC) activation, but it is not PI3K-dependent, while late Akt phosphorylation is PI3K-dependent36. Psm2 showed no influence on early (60 s) AktSer473 phosphorylation, but inhibited late (180 s) AktSer473 phosphorylation, suggesting that Psm2 exerted antiplatelet effects by affecting PI3K signaling but not PLC inhibition. All of these results suggest that Psm2 can block PI3K signaling and thereby inhibit platelet aggregation.
Various PI3K isoforms are expressed in platelets, including the class Ia isoforms, PI3Kα and PI3Kβ, and the class Ib isoform, PI3Kγ37. It has been demonstrated that PI3Kβ (not α or γ) plays a significant role in regulating the activation and adhesion of the GPIIb/IIIa receptor, which is required for platelet spreading and sustained platelet aggregation, especially when platelet responses are induced by ADP38,39. Molecular docking showed that Psm2 can interact with PI3Kβ by forming hydrogen bonds with the Lys776 and Lys799 residues of its active site, suggesting that Psm2 influences the PI3K/Akt signaling in platelets via inhibiting PI3Kβ.
In an arterio-venous shunt model, developing thrombi contain aggregated platelets that adhere to the thrombogenic silk thread and fibrin1. Thus, arterial thrombus formation models have been considered classical assays for determining the antithrombotic activities of an antiplatelet “drug”. Our studies showed that Psm2 markedly decreased the wet and dry thrombus weight in the arterial thrombus formation model in a dose dependent manner, and at dose of 10 mg/kg, the effect was comparable with that of aspirin (50 mg/kg). The results clearly indicate that Psm2 has an antithrombotic effect and an efficacy stronger than that of aspirin, a traditional antiplatelet agent, and may be a candidate for an antithrombotic agent.
A suitable risk-benefit profile (for the antithrombotic effects and the side effects) is extremely important for the clinical application of antiplatelet drugs. We evaluated the cytotoxicity of Psm2 and the bleeding risk of administering Psm2. These results indicated that Psm2 had no cytotoxicity, even at a concentration of 6.0 mg/mL, at which the cell survival rate was more than 90%. It also showed little bleeding risk, even at a dose of 10 mg/kg, which had potent antithrombotic effects. Additionally, the bleeding risk of Psm2 administration was lower than that of aspirin. These results demonstrated that Psm2 is a safe and effective compound for antithrombotic therapy.
Taken together, these findings show that Psm2 effectively inhibited platelet aggregation in vitro and ex vivo, as well as attenuated thrombus formation in vivo with a minimal bleeding risk. Mechanistically, Psm2 inhibited PI3K signaling and interacted with PI3Kβ, a promising target for the treatment of thrombotic diseases. Therefore, Psm2 is an antithrombotic molecule that may be developed for the management of thrombotic diseases.
Abbreviations
ELISA, enzyme-linked immunosorbent assay; MAPK, mitogen activated protein kinase; PI3K, phosphatidylinositol 3-kinase; PLCγ2, phospholipase Cγ2; TXA2, thromboxane A2; GPIIb/IIIa, glycoprotein IIb–IIIa; APTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time; A–V, arterio-venous; ICR, Institute of Cancer Research; SD, Sprague-Dawley; PRP, platelet-rich plasma; PPP, platelet-poor plasma; PVDF, poly vinylidene difluoride; MOE, Molecular Operating Environment; MM/GBVI, Molecular Mechanics Generalized Born/Volume Integral.
Author contribution
Xing-li SU and Wen SU performed the research and wrote the manuscript; Ying WANG performed the partial experiments; Xin MING provided suggestions about this study and modified the manuscript; Yue-hu WANG provided the compound Psm2 and revised the manuscript; and Yi KONG conceived the study, designed the research and modified the manuscript. All authors read and approved the final manuscript.
References
Chen M, Ye X, Ming X, Chen Y, Wang Y, Su X, et al. A novel direct factor Xa inhibitory peptide with anti-platelet aggregation activity from Agkistrodon acutus venom hydrolysates. Sci Rep 2015; 5: 10846.
Ye X, Chen M, Chen Y, Su X, Wang Y, Su W, et al. Isolation and characterization of a novel antithrombotic peptide from enzymatic hydrolysate of Agkistrodon acutus venom. Int J Pert Res Ther 2015; 21: 343–51.
Peng L, Xu X, Shen D, Zhang Y, Song J, Yan X, et al. Purification and partial characterization of a novel phosphodiesterase from the venom of Trimeresurus stejnegeri: inhibition of platelet aggregation. Biochimie 2011; 93: 1601–9.
Lu WQ, Qiu Y, Li TJ, Tao X, Sun LN, Chen WS . Antiplatelet and antithrombotic activities of timosaponin B-II, an extract of Anemarrhena asphodeloides. Clin Exp Pharmacol P 2011; 38: 430–4.
Michelson AD . Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov 2010; 9: 154–69.
Pan C, Wei X, Ye J, Liu G, Zhang S, Zhang Y, et al. BF066, a novel dual target antiplatelet agent without significant bleeding. PLoS One 2012; 7: e40451.
Johnson S . Known knowns and known unknowns: risks associated with combination antithrombotic therapy. Thromb Res 2008; 123: S7–11.
Jackson S, Yap C, Anderson K . Phosphoinositide 3-kinases and the regulation of platelet function. Biochem Soc T 2004; 32: 387–92.
Chen J, De S, Damron DS, Chen WS, Hay N, Byzova TV . Impaired platelet responses to thrombin and collagen in AKT-1–deficient mice. Blood 2004; 104: 1703–10.
Huang Z, Zeng C, Zhu L, Jiang L, Li N, Hu H . Salvianolic acid A inhibits platelet activation and arterial thrombosis via inhibition of phosphoinositide 3-kinase. J Thromb Haemost 2010; 8: 1383–93.
Sheu J, Hung W, Wu C, Lee Y, Yen M . Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa, on platelet plug formation in in vivo experiments. Br J Haematol 2000; 110: 110–5.
Wu ZY, Zhou TY, Xiao PG . Xinghua Bencao Gangyao. v 3. Shanghai: Shanghai Scientific and Technological Press; 1990. p 626–31.
Zhang XC, Nooteboom HP, Kato M . Selaginellaceae. In: Wu ZY, Raven PH, Hong DY, editors. Flora of China. v 2–3. Pteridophytes. Beijing: Beijing and Missouri Botanical Garden Press; 2013. p 37–46.
Wang YH, Long CL, Yang FM, Wang X, Sun QY, Wang HS, et al. Pyrrolidinoindoline alkaloids from Selaginella moellendorfii. J Nat Prod 2009; 72: 1151–4.
Klafke JZ, da Silva MA, Rossato MF, Trevisan G, Walker CIB, Leal CAM, et al. Antiplatelet, antithrombotic, and fibrinolytic activities of Campomanesia xanthocarpa. Evid-Based Compl Alt 2012; 2012: 1–8.
Shi S, Zhou H, Zhang Y, Huang K . Hyphenated HSCCC–DPPH for rapid preparative isolation and screening of antioxidants from Selaginella moellendorffii. Chromatographia 2008; 68: 173–8.
Fan HY, Fu FH, Yang MY, Xu H, Zhang AH, Liu K . Antiplatelet and antithrombotic activities of salvianolic acid A. Thromb Res 2010; 126: e17–22.
Huang J, Wang S, Luo X, Xie Y, Shi X . Cinnamaldehyde reduction of platelet aggregation and thrombosis in rodents. Thromb Res 2007; 119: 337–42.
Gadi D, Bnouham M, Aziz M, Ziyyat A, Legssyer A, Legrand C, et al. Parsley extract inhibits in vitro and ex vivo platelet aggregation and prolongs bleeding time in rats. J Ethnopharmacol 2009; 125: 170–4.
Umar A, Guerin V, Renard M, Boisseau M, Garreau C, Begaud B, et al. Effects of armagnac extracts on human platelet function in vitro and on rat arteriovenous shunt thrombosis in vivo. Thromb Res 2003; 110: 135–40.
Umetsu T, Sanai K . Effect of 1-methyl-2-mercapto-5-(3-pyridyl)-imidazole (KC-6141), an anti-aggregating compound, on experimental thrombosis in rats. Thromb Haemost 1978; 39: 74–83.
Zhu L, Zhao L, Wang H, Wang Y, Pan D, Yao J, et al. Oroxylin A reverses P-glycoprotein-mediated multidrug resistance of MCF7/ADR cells by G2/M arrest. Toxicol Lett 2013; 219: 107–15.
Zhu B, Zhao L, Zhu L, Wang H, Sha Y, Yao J, et al. Oroxylin a reverses CAM-DR of HEPG2 cells by suppressing integrin β1 and its related pathway. Toxicol Appl Pharm 2012; 259: 387–94.
Liu Y, Jennings NL, Dart AM, Du XJ . Standardizing a simpler, more sensitive and accurate tail bleeding assay in mice. World J Exp Med 2012; 2: 30–6.
Yang T, Jia M, Mei Q, Shang P . Effects of Angelica polysaccharide on blood coagulation and platelet aggregation. Zhong Yao Cai 2002; 25: 344–5.
Blue R, Murcia M, Karan C, Jiroušková M, Coller BS . Application of high-throughput screening to identify a novel αIIb-specific small-molecule inhibitor of αIIbβ3-mediated platelet interaction with fibrinogen. Blood 2008; 111: 1248–56.
Chadderdon RC, Cappello M . The hookworm platelet inhibitor: functional blockade of integrins GPIIb/IIIa (αIIbβ3) and GPIa/IIa (α2β1) inhibits platelet aggregation and adhesion in vitro. J Infect Dis 1999; 179: 1235–41.
Da Silva M, Lucena S, Aguilar I, Rodríguez-Acosta A, Salazar AM, Sánchez EE, et al. Anti-platelet effect of cumanastatin 1, a disintegrin isolated from venom of South American Crotalus rattlesnake. Thromb Res 2009; 123: 731–9.
Kong Y, Wang Y, Yang W, Xie Z, Li Z . LX0702, a novel snake venom peptide derivative, inhibits thrombus formation via affecting the binding of fibrinogen with GPIIb/IIIa. J Pharmacol Sci 2015; 127: 462–6.
Ma D, Xu X, An S, Liu H, Yang X, Andersen JF, et al. A novel family of RGD-containing disintegrin (Tablysin-15) from the salivary gland of the horsefly Tabanus yao targets integrins αIIbβ3 and αVβ3 and inhibits platelet aggregation and angiogenesis. Thromb Haemost 2011; 105: 1032–45.
Prevost N, Woulfe D, Tanaka T, Brass LF . Interactions between Eph kinases and ephrins provide a mechanism to support platelet aggregation once cell-to-cell contact has occurred. Proc Natl Acad Sci U S A 2002; 99: 9219–24.
Yi W, Li Q, Shen J, Ren L, Liu X, Wang Q, et al. Modulation of platelet activation and thrombus formation using a Pan-PI3K inhibitor S14161. PLoS One 2014; 9: e102394.
Pinson JA, Zheng Z, Miller MS, Chalmers DK, Jennings IG, Thompson PE . l-Aminoacyl-triazine derivatives are isoform-selective PI3Kβ inhibitors that target nonconserved Asp862 of PI3Kβ. ACS Med Chem Lett 2012; 4: 206–10.
Li Z, Delaney MK, O'Brien KA, Du X . Signaling during platelet adhesion and activation. Arterioscl Throm Vas 2010; 30: 2341–9.
Gao C, Boylan B, Bougie D, Gill JC, Birenbaum J, Newman DK, et al. Eptifibatide-induced thrombocytopenia and thrombosis in humans require FcγRIIa and the integrin β3 cytoplasmic domain. J Clin Invest 2009; 119: 504–11.
Savage B, Saldívar E, Ruggeri ZM . Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 1996; 84: 289–97.
Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, et al. PI 3-kinase p110β: a new target for antithrombotic therapy. Nat Med 2005; 11: 507–14.
Francischetti IM . Platelet aggregation inhibitors from hematophagous animals. Toxicon 2010; 56: 1130–44.
Hyun KW, Jeong SC, Lee DH, Park JS, Lee JS . Isolation and characterization of a novel platelet aggregation inhibitory peptide from the medicinal mushroom, Inonotus obliquus. Peptides 2006; 27: 1173–8.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No 81273375), the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Jiangsu Provincial Qing Lan Project.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Su, Xl., Su, W., Wang, Y. et al. The pyrrolidinoindoline alkaloid Psm2 inhibits platelet aggregation and thrombus formation by affecting PI3K/Akt signaling. Acta Pharmacol Sin 37, 1208–1217 (2016). https://doi.org/10.1038/aps.2016.52
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.52
Keywords
This article is cited by
-
Fibroblast growth factor-21 as a novel metabolic factor for regulating thrombotic homeostasis
Scientific Reports (2022)
-
Pharmacological actions of miltirone in the modulation of platelet function
Acta Pharmacologica Sinica (2019)
-
Amides, Isoquinoline Alkaloids and Dipeptides from the Aerial Parts of Piper mullesua
Natural Products and Bioprospecting (2018)