Abstract
Aim:
Paeoniflorin has shown to attenuate bleomycin-induced pulmonary fibrosis (PF) in mice. Because the epithelial-mesenchymal transition (EMT) in type 2 lung endothelial cells contributes to excessive fibroblasts and myofibroblasts during multiple fibrosis of tissues, we investigated the effects of paeoniflorin on TGF-β mediated pulmonary EMT in bleomycin-induced PF mice.
Methods:
PF was induced in mice by intratracheal instillation of bleomycin (5 mg/kg). The mice were orally treated with paeoniflorin or prednisone for 21 d. After the mice were sacrificed, lung tissues were collected for analysis. An in vitro EMT model was established in alveolar epithelial cells (A549 cells) incubated with TGF-β1 (2 ng/mL). EMT identification and the expression of related proteins were performed using immunohistochemistry, transwell assay, ELISA, Western blot and RT-qPCR.
Results:
In PF mice, paeoniflorin (50, 100 mg·kg−1·d−1) or prednisone (6 mg·kg−1·d−1) significantly decreased the expression of FSP-1 and α-SMA, and increased the expression of E-cadherin in lung tissues. In A549 cells, TGF-β1 stimulation induced EMT, as shown by the changes in cell morphology, the increased cell migration, and the increased vimentin and α-SMA expression as well as type I and type III collagen levels, and by the decreased E-cadherin expression. In contrast, effects of paeoniflorin on EMT disappeared when the A549 cells were pretreated with TGF-β1 for 24 h. TGF-β1 stimulation markedly increased the expression of Snail and activated Smad2/3, Akt, ERK, JNK and p38 MAPK in A549 cells. Co-incubation with paeoniflorin (1–30 μmol/L) dose-dependently attenuated TGF-β1-induced expression of Snail and activation of Smad2/3, but slightly affected TGF-β1-induced activation of Akt, ERK, JNK and p38 MAPK. Moreover, paeoniflorin markedly increased Smad7 level, and decreased ALK5 level in A549 cells.
Conclusion:
Paeoniflorin suppresses the early stages of TGF-β mediated EMT in alveolar epithelial cells, likely by decreasing the expression of the transcription factors Snail via a Smad-dependent pathway involving the up-regulation of Smad7.
Similar content being viewed by others
Introduction
Idiopathic pulmonary fibrosis (IPF) is a devastating, irreversible and fatal lung disease with a global incidence of 2–29 per 100 000 people every year1,2. The major characteristics of IPF include alveolar catarrh, abnormal proliferation and transformation of fibroblasts, excessive secretion and deposition of extracellular matrix (ECM), the subsequent irreversible destruction of the lung architecture and fibrosis3,4. The precise pathogenesis of IPF is not yet completely understood, but effective intervention can be achieved by inhibiting the synthesis of ECM and promoting its degradation in lung tissues.
ECM includes collagen (the major component), proteoglycan, fibronectin, elastin and other matrix components. Type I collagen is the most common form of ECM collagen5,6. Fibroblasts and myofibroblasts are the major effector cells that synthetize and secret type I collagen in lung tissues. Moreover, myofibroblasts—α-smooth muscle actin (α-SMA)-expressing fibroblasts—have been demonstrated to be the main source of type I collagen, and they have a greater ability to produce type I collagen than fibroblasts7. The excessive production of fibroblasts and myofibroblasts results from 1) the proliferation and differentiation of resident mesenchymal cells, 2) EMT (epithelial-mesenchymal transition) in type II lung epithelial cells8, and 3) the recruitment of bone marrow-derived progenitor cells. In fact, in a bleomycin-induced lung fibrosis mouse model, approximately one-third of the fibroblasts in lung tissues were derived from type II lung epithelial cells that underwent EMT9.
Paeoniflorin (Figure 1), a pinane monoterpene glucoside found in the root of Paeonia lactiflora Pall, has been reported to have anti-inflammatory and immunomodulatory properties10,11,12. Recently, we demonstrated that paeoniflorin substantially prevented pulmonary fibrosis (PF) in bleomycin-treated mice by suppressing the synthesis of type I collagen in lung tissues13. To identify the mechanism by which paeoniflorin suppresses the synthesis of type I collagen in PF, the present study was aimed at investigating the effect of paeoniflorin on TGF-β mediated pulmonary EMT using in vivo and in vitro assays.
The chemical structure of paeoniflorin.
Materials and methods
Chemicals and reagents
Paeoniflorin (purity >95%, MW: 480.45, dissolved in DMSO to a final concentration lower than 0.1%) was purchased from Nanjing Zelang Medical Technology Co, Ltd (Nanjing, China); prednisone acetate was purchased from Zhejiang Xianju Pharmaceutical Co, Ltd (Taizhou, China); bleomycin hydrochloride (BLM) was purchased from Nippon Kayaku (Tokyo, Japan); RPMI-1640 and fetal bovine serum (FBS) were purchased from HyClone (Logan, USA); recombinant human TGF-β1 was purchased from R&D Systems (Minneapolis, USA); E-cadherin, Smad2/3, p-Smad2 and p-Smad3 antibodies were purchased from Cell Signaling Technology (Boston, MA, USA); α-SMA antibodies were purchased from Epitomics (Burlingame, CA, USA); FSP-1, Smad7, ALK5 and vimentin antibodies were purchased from Bioworld Technology, Inc (Minneapolis, USA); Akt, p-Akt; JNK, p-JNK, ERK, p-ERK, p38, p-p38 and GAPDH antibodies were purchased from Kangchen Biotech (Shanghai, China); type I collagen ELISA kits were purchased from Abcam (Cambridge, UK); iScript cDNA synthesis kits and SsoFast EvaGreen Supermix were purchased from Bio-Rad (Hercules, USA); and TRIzol reagent was purchased from TransGen Biotech (Beijing, China). All other chemicals and reagents used were of analytical grade.
Animals
Male ICR mice weighing 22±2 g were purchased from the Comparative Medicine Centre of Yangzhou University (Yangzhou, China). The mice were allowed to acclimatize to the laboratory environment for at least 7 d at a constant temperature (23±2 °C) before being used. Food and water were provided ad libitum. All animal care and use protocols complied with the Provisions and General Recommendation of the Chinese Experimental Animals Administration Legislation. The study protocol was approved by the Institutional Ethical Committee of China Pharmaceutical University.
PF induced by BLM in mice
Briefly, a PF model was established using an intratracheal instillation of BLM (5 mg/kg in saline) in mice13. Then, the mice were fixed on a board and swung using a circular motion for 2 min to ensure that the BLM was well distributed in the lungs. On d 1, the mice were randomly separated into a normal group, a model group, paeoniflorin (25, 50 and 100 mg/kg)-treated groups, and a prednisone (6 mg/kg)-treated group. Paeoniflorin and prednisone, dissolved in water, were orally administered for 21 consecutive days at a volume of 0.1 mL/10 g body weight. On d 21, the mice were sacrificed using an excess of chloral hydrate hydrochloride anesthesia, and the lower left sides of the lung tissues were prepared for immunohistochemistry. Other lung tissues were stored at −70 °C.
Immunohistochemistry
The lower left sides of lung tissues were fixed in 10% formalin, embedded in paraffin, sectioned, and then prepared for immunohistochemical staining. Sections were incubated with anti-FSP-1, anti-α-SMA or anti-E-cadherin antibodies for 24 h at 4 °C, and then incubated with goat anti-rabbit IgG secondary antibodies for 1 h at 37 °C. After incubation with 3,3′-diaminobenzidine tetrahydrochloride and hydrogen peroxide, the sections were counterstained with hematoxylin.
Cell culture
Human type II alveolar epithelial cell lines (A549), obtained from the American Type Culture Collection (ATCC), were cultured in RPMI-1640 containing 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin and maintained at 37 °C in a 5% CO2 humidified atmosphere. For EMT induction, A549 cells (5×105 cells/well) were seeded into 6-well plates and incubated with serum-free RPMI-1640 for 2 h. Then, the cells were stimulated with TGF-β1 (2 ng/mL) for 48 h14. Cell morphology was observed and recorded under an inverted microscope (magnification, 200×).
Cell viability assay
The cytotoxic effects of paeoniflorin were evaluated using a conventional MTT assay. Briefly, A549 cells (1×105 cells/well) were seeded and plated into 96-well plates and then incubated in serum-free medium for 2 h. Then, the cells were incubated with paeoniflorin (1, 3, 10, or 30 μmol/L) in the presence or absence of TGF-β1 (2 ng/mL) for 20 h. Then, 20 μL of MTT (5 mg/mL) was added to each well, and the cells were incubated for an additional 4 h. Subsequently, the supernatants were removed, and the formazan crystals were dissolved in DMSO (150 μL). Absorbance was measured at 490 nm using a Model 1500 Multiskan Spectrum Microplate Reader (Thermo, Waltham, MA, USA).
Cell migration assay
A549 cell migration assays were performed using a transwell Boyden chamber (Corning Costar, Cambridge, MA, USA) containing a polycarbonate filter with a pore size of 8 μm. Cells were seeded in the upper well of each chamber, and the chambers were inserted into 24-well plates. Cells were incubated with serum-free medium for 2 h and then treated with culture medium containing 2% NBCS in the presence of TGF-β1 (2 ng/mL) or TGF-β1 (2 ng/mL) plus paeoniflorin (1, 3, 10, and 30 μmol/L) for 6 h. Then, the non-migrated cells on the upper surface of the membrane were removed by washing the membranes in PBS, and the migrated cells on the bottom surface of the transwell membrane were stained with 5% Cresyl violet for 15 min.
Western blot analysis
A549 cells were seeded into 6-well plates and incubated with serum-free medium for 2 h. Subsequently, cells were treated with paeoniflorin (1, 3, 10, and 30 μmol/L) for 24 h and then stimulated using TGF-β1 (2 ng/mL) for 0, 5, 10, 20, 30, and 60 min. The cells were washed twice with ice-cold phosphate-buffered saline (pH 7.2), and proteins were extracted with lysis buffer for 30 min on ice. The extracts were harvested using centrifugation at 12 000 rounds per minute for 5 min at 4 °C. The protein concentrate was quantified using a Bradford assay. The protein samples (20 μg per lane) were loaded and run using 12% SDS-PAGE and then transferred to PVDF membranes. The membranes were blocked for 2 h at room temperature in 10% nonfat milk in TBS-Tween 20 (0.1%) and then incubated with the corresponding antibodies. Finally, the membranes were incubated in ECL reagent for 2–10 min and then exposed to X-ray film.
ELISA
A549 cells were seeded into 6-well plates and incubated with serum-free medium for 2 h. Subsequently, cells were treated with TGF-β1 (2 ng/mL) or TGF-β1 (2 ng/mL) plus paeoniflorin (1, 3, 10, and 30 μmol/L) for 48 h. The supernatants were collected and stored at −20 °C. The level of type I collagen was measured using enzyme-linked immunosorbent assays (ELISA).
Real-time quantitative polymerase chain reaction (RT-qPCR)
A549 cells were seeded into 6-well plates and incubated with serum-free medium for 2 h. Subsequently, cells were treated with TGF-β1 (2 ng/mL) or TGF-β1 (2 ng/mL) plus paeoniflorin (1, 3, 10, and 30 μmol/L) for 24 h. Total RNA was extracted using TRIzol (0.5 mL) according to the manufacturer's instructions. The concentrations were determined using optical density measurements at 260 nm on a spectrophotometer. RNA (2 μg) was used to perform reverse transcriptase reactions according to the manufacturer's instructions. Then, 20-μL reaction mixtures containing Ssofast Evagreen Supermix, nuclease-free water, forward primer, reverse primer and cDNA were transferred to different PCR tubes. Melting curves were determined for every run to ensure that only the correct product was amplified. The Ct values were normalized to the corresponding values of GAPDH. The primers used for RT-qPCR are shown in Table 1.
Statistical analysis
All data are expressed as the mean±SD. Statistical significance was evaluated using one-way analysis of variance (ANOVA) followed by post-hoc Tukey's test. P values less than 0.05 (P<0.05) were considered to be significant.
Results
Effect of paeoniflorin on BLM-induced EMT in mouse lung tissues
During the course of PF, type II lung epithelial cells are stimulated by TGF-β and other factors to undergo EMT and transform into fibroblasts and myofibroblasts. These cells secret large amounts of ECM, which destroys the lung architecture and aggravate the fibrotic process. FSP-1 and α-SMA are specific markers of fibroblasts and myofibroblasts, respectively. Fibroblasts and myofibroblasts are primarily responsible for the excessive production and deposition of ECM in PF. Emerging evidence indicates that type II lung epithelial cells, which express the biomarker E-cadherin, can undergo EMT to eventually transform into myofibroblasts in PF.
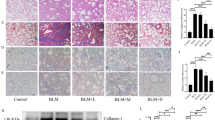
To assess whether paeoniflorin affects pulmonary EMT in BLM-induced PF, we investigated the expression of FSP-1, α-SMA and E-cadherin in the lung tissues of mice on d 21 after treatment with BLM. As shown in Figure 2 and Supplementary Figure S1, FSP-1- and α-SMA-positive areas were considerably increased in the lung tissues of BLM-treated mice, while paeoniflorin (50 and 100 mg/kg) and prednisone (6 mg/kg) significantly decreased the FSP-1- and α-SMA-positive areas. By contrast, treatment with BLM resulted in a dramatic decrease in the E-cadherin-positive area in the lung tissues of mice. Both paeoniflorin (25, 50, and 100 mg/kg) and prednisone (6 mg/kg) nearly completely restored the E-cadherin-positive area (Figure 2C).
The effects of paeoniflorin (Pae) and prednisone (Pred) on the expression levels of FSP-1, α-SMA and E-cadherin on bleomycin-induced fibrosis in mouse lung tissues. On d 21 after BLM instillation, mice were sacrificed using an excess of chloral hydrate hydrochloride anesthesia, and their lung tissues were isolated. The expression and localization of FSP-1, α-SMA and E-cadherin protein in the lower left sides of lung tissues were examined using immunohistochemistry. n=6. ##P<0.01 vs normal. *P<0.05, **P<0.01 vs model.
Effect of paeoniflorin on the viability of A549 cells
To investigate whether paeoniflorin is cytotoxic in alveolar epithelial cells, A549 cells (human type II alveolar epithelial cells) were treated with paeoniflorin (1, 3, 10, and 30 μmol/L) in the absence or presence of TGF-β1 (2 ng/mL) for 24 h. MTT assays showed that paeoniflorin induced no observable cell cytotoxicity under the tested concentrations (Figure 3).
The effects of paeoniflorin (Pae) on the cell viability in A549 cells. Cell viability was detected using MTT assays. A549 cells were seeded into 96-well plates and then incubated with serum-free RPMI-1640 for 2 h. Then, cells were incubated with Pae (1, 3, 10 and 30 μmol/L) in the presence or absence of TGF-β1 (2 ng/mL) for 20 h, and their viability was detected using MTT assays as described in the Methods section. (A) Cell viability was analyzed in A549 cells treated with Pae. (B) Cell viability was analyzed in A549 cells treated with Pae and TGF-β1. Data are expressed as the mean±SD. n=3.
Effect of paeoniflorin on EMT induced by TGF-β1 in A549 cells
To further investigate the effects and characteristics of paeoniflorin on TGF-β-mediated EMT in alveolar epithelial cells, in vitro experiments were performed in A549 cells using TGF-β1 as a stimulant. In the absence or presence of paeoniflorin, A549 cells were treated with TGF-β1 (2 ng/mL) for 48 h. Changes in morphology, protein marker expression (E-cadherin, Vimentin and α-SMA) and type I collagen secretion were examined. As shown in Figure 4 and Supplementary Figure S2, in contrast to normal cells, TGF-β1-treated cells changed from a cobblestone-like monolayer of epithelial cells into spindle-shaped mesenchymal cells and expressed higher migratory potential. Meanwhile, the expression of E-cadherin decreased while the expressions of Vimentin and α-SMA increased. The levels of type I and III collagen in cells steeply increased. Paeoniflorin (3, 10, and 30 μmol/L) significantly inhibited these morphological changes and migration in A549 cells, up-regulated the expression of E-cadherin, and decreased the expression of Vimentin and α-SMA.
The effects of different concentrations of paeoniflorin (Pae) on TGF-β1-induced epithelial-mesenchymal transition in A549 cells. A549 cells were seeded into plates and incubated with serum-free RPMI 1640 for 2 h. Then, the cells were incubated with TGF-β1 (2 ng/mL) or TGF-β1 (2 ng/mL) plus Pae (1, 3, 10 and 30 μmol/L) for the indicated intervals. (A) The expression levels of E-cadherin, Vimentin and α-SMA were detected using Western blot analysis. (B) The amounts of type I and type III collagen in the cell supernatants were detected using ELISA and Real-Time Quantitative PCR assays. Data are expressed as the means±SD. n=3. ##P<0.01 vs normal. *P<0.05, **P<0.01 vs TGF-β1.
Additionally, the levels of type I and III collagen were considerably decreased after paeoniflorin treatment. The percentage decrease induced by treatment with paeoniflorin on the mRNA levels of type I and III collagen were 54.3% and 65.7% for 10 μmol/L paeoniflorin and 56.1% and 67.3% for 30 μmol/L paeoniflorin.
Time course of paeoniflorin-induced inhibition of EMT in A549 cells
To determine which stage paeoniflorin primarily affects, A549 cells were cultured with paeoniflorin (10 μmol/L) for different intervals after stimulation with TGF-β1 (2 ng/mL). Changes in cell morphology, protein marker expression and type I collagen secretion were recorded. As shown in Figure 5, after stimulation with TGF-β1 for 48 h, a considerable number of the A549 cells converted into spindle-shaped mesenchymal cells, and this alteration in morphology was accompanied by decreased expression of E-cadherin, increased expression of Vimentin and α-SMA, and increased secretion of type I collagen. Paeoniflorin significantly prevented EMT in A549 cells when it was added at the same time as or at 6 and 12 h after TGF-β1 stimulation. If added at 24 h or 36 h after TGF-β1 stimulation, paeoniflorin had little effect. When A549 cells were treated with paeoniflorin (10 μmol/L) for 6 h and 12 h, E-cadherin expression increased approximately 7.5- and 5-fold, respectively, and Vimentin expression and α-SMA expression were inhibited by 37.7% and 14.8% (for 6 h) and 44.2% and 18.9% (for 12 h), respectively.
Time course of the effect of paeoniflorin (Pae) on TGF-β1-induced epithelial-mesenchymal transition in A549 cells. A549 cells were seeded into plates and incubated with serum-free RPMI-1640 for 2 h. Then, the cells were incubated with TGF-β1 (2 ng/mL) or TGF-β1 (2 ng/mL) plus Pae (1, 3, 10 and 30 μmol/L) for the indicated intervals. (A) Cell morphology was recorded using an inverted microscope (magnification 200×). (B) The expression levels of E-cadherin, Vimentin and α-SMA were detected using Western blot analysis, as described in the Methods section. GAPDH was used as the internal control. (C) The protein levels of E-cadherin, Vimentin and α-SMA were determined using Real-Time Quantitative PCR, as described in the Methods section. (D) The cell supernatants were collected, and the amount of type I collagen was detected using ELISA, as described in the Methods section. The data are expressed as the mean±SD. n=3. ##P<0.01 vs normal. *P<0.05, **P<0.01 vs TGF-β1.
Effect of paeoniflorin on the expression of EMT-related transcription factors in A549 cells
Deficiency in E-cadherin is a key event in the EMT process. Many transcription factors (eg, Snail, Slug, Twist, ZEB1 and ZEB2) have been found to participate in the regulation of E-cadherin expression. To gain insight into the mechanisms by which paeoniflorin prevents E-cadherin deficiency in TGF-β1-induced A549 cells, its effects on the expression levels of the transcription factors mentioned above were examined using RT-qPCR. As shown in Figure 6A, the mRNA expression levels of Snail, Slug, Twist, ZEB1 and ZEB2 were significantly increased by treating cells with TGF-β1 (2 ng/mL) for different intervals, peaking at 24 h and then declining. Then, A549 cells were exposed to different concentrations of paeoniflorin and cultured for 24 h in the presence of TGF-β1. As shown in Figure 6B, paeoniflorin (3, 10, and 30 μmol/L) treatment dramatically inhibited the expression of Snail mRNA but only slightly affected the expression of Slug, Twist, ZEB1 and ZEB2 mRNA. At a concentration of 3, 10, or 30 μmol/L, paeoniflorin inhibited the mRNA expression of Snail by 81.5%, 86.8%, and 60.5%, respectively. These results suggest that the down-regulation of Snail expression might be the key mechanism by which paeoniflorin prevents E-cadherin deficiency in A549 cells.
The effects of paeoniflorin (Pae) on the expression levels of transcription factors in TGF-β1-stimulated A549 cells. A549 cells were seeded into plates and incubated with serum-free RPMI-1640 for 2 h. Then, the cells were incubated with Pae (1, 3, 10 and 30 μmol/L) in the presence or absence of TGF-β1 (2 ng/mL) for the indicated time interval. The expression levels of Snail, Slug, ZEB1, ZEB2 and Twist mRNAs were measured using Real-Time Quantitative PCR, as described in the Methods section. (A) Time course showing Snail, Slug, ZEB1, ZEB2 and Twist mRNA expression. (B) The effects of Pae (1, 3, 10, and 30 μmol/L) on the expression levels of Snail, Slug, ZEB1, ZEB2 and Twist mRNAs. The data are expressed as the mean±SD, n=3. *P<0.05, **P<0.01 vs normal (in Figure 6A); #P<0.05, ##P<0.01 vs normal. *P<0.05, **P<0.01 vs TGF-β1 (in Figure 6B).
Effect of paeoniflorin on the activation of the Smad-dependent pathway in A549 cells
The expression of Snail is regulated by both Smad-dependent and Smad-independent signaling pathways. To determine the mechanisms by which paeoniflorin inhibits the expression of Snail mRNA, the effects of paeoniflorin on the activation of the Smad pathway were first investigated. A549 cells were exposed to TGF-β1 for 5, 10, 20, 30 and 60 min. The levels of phosphorylated Smad2 and Smad3 were detected using Western blot analysis. As shown in Figure 7A, phosphorylated Smad2 and Smad3 were significantly increased by TGF-β1 (2 ng/mL) stimulation, peaking at 30 min. Paeoniflorin (3, 10, and 30 μmol/L) markedly inhibited the phosphorylation of both Smad2 and Smad3, inhibiting Smad2 phosphorylation by 38.2%, 60.2% and 72.6%, respectively, and Smad3 phosphorylation by 46.2%, 68.2% and 73.5%, respectively (Figure 7B). These findings suggest that paeoniflorin might regulate the expression of Snail through a Smad-dependent pathway.
The effects of paeoniflorin (Pae) on the activation of the Smad-dependent pathway in A549 cells stimulated using TGF-β1. A549 cells were seeded into plates and incubated with serum-free RPMI-1640 for 2 h. Then, the cells were incubated with Pae (1, 3, 10 and 30 μmol/L) and stimulated using TGF-β1 (2 ng/mL) for the indicated time interval. The expression levels of p-Smad2 and p-Smad3 were detected using Western blot analysis. GAPDH was used as the internal control. (A) Time course showing p-Smad2 and p-Smad3 expression. (B) The effects of Pae (1, 3, 10, and 30 μmol/L) on p-Smad2 and p-Smad3 expression levels. The data are expressed as the mean±SD, n=3. #P<0.05, ##P<0.01 vs normal. *P<0.05, **P<0.01 vs TGF-β1.
Effect of paeoniflorin on the Smad-independent pathway in A549 cells
Next, we investigated the effect of paeoniflorin on the activation of the Smad-independent pathway, including the PI3K/Akt and MAPK pathways. A549 cells were exposed to TGF-β1 (2 ng/mL) for 5, 10, 20, 30 and 60 min, respectively. The levels of phosphorylated Akt, ERK, JNK and p38 MAPK were detected using Western blot analysis. As shown in Figure 8A, the levels of phosphorylated Akt, ERK, JNK and p38 MAPK were significantly increased by TGF-β1 stimulation, peaking at 30 min. Then, we cultured A549 cells with paeoniflorin in the presence of TGF-β1 for 30 min. The results showed that paeoniflorin (1, 3, 10, and 30 μmol/L) only slightly affected the phosphorylation of Akt, ERK, JNK and p38 MAPK, indicating that paeoniflorin does not down-regulate the expression of Snail by a Smad-independent pathway (Figure 8B).
The effects of paeoniflorin (Pae) on the activation of the Smad-independent pathway and the levels of Smad 7 and ALK5 in A549 cells stimulated by TGF-β1. A549 cells were seeded into plates and incubated with serum-free RPMI-1640 for 2 h. Then, the cells were incubated with Pae (1, 3, 10 and 30 μmol/L) and stimulated using TGF-β1 (2 ng/mL) for the indicated time interval. The expression levels of p-Akt/Akt, p-p38/p38, p-ERK/ERK and p-JNK/JNK were detected using Western blot analysis. GAPDH was used as the internal control. (A) Time course showing p-Akt/Akt, p-ERK/ERK, p-JNK/JNK and p-p38/p38 protein expression levels. (B) The effects of Pae (1, 3, 10, and 30 μmol/L) on p-Akt/Akt, p-Erk/Erk, p-JNK/JNK and p-p38/p38 protein expression levels. (C) The effects of Pae (1, 3, 10, and 30 μmol/L) on Smad7 and ALK5 levels. The data are expressed as the mean±SD, n=3. ##P<0.01 vs Normal, *P<0.05 vs TGF-β1.
Effect of paeoniflorin on the levels of Smad7 and ALK5 in A549 cells
Previous reports have indicated that Smad7 regulates the canonical TGF-β/Smad pathway by associating with ALK5, the type I receptor of TGF-β. Therefore, we next investigated the effect of paeoniflorin on the TGF-β1-induced levels of Smad7 and ALK5 in A549 cells. TGF-β1 stimulation increased ALK5 expression and decreased Smad7 expression in A549 cells. However, paeoniflorin (3, 10, and 30 μmol/L) treatment resulted in the significant down-regulation in ALK5 and the up-regulation of Smad7 compared to the TGF-β1-only group (Figure 8C). Treatment with paeoniflorin (3, 10, and 30 μmol/L) inhibited ALK5 by 47.5%, 45.8% and 57.6%, respectively, and increased Smad7 by 0.52-, 3.15- and 2.75-fold, respectively.
Discussion
EMT is a biological process by which epithelial cells lose their cell-cell adhesions and acquire the characteristics of mesenchymal cells15. EMT can be classified into three subtypes based on the situation under which it occurs. Type 1 EMT involves primitive epithelial cells transitioning into motile mesenchymal cells during embryogenesis and organogenesis. Type 2 EMT involves endothelial cells transitioning into resident tissue fibroblasts during multiple fibrosis in tissues, including pulmonary fibrosis. Type 3 EMT involves the transitioning of epithelial carcinoma cells in primary nodules into metastatic tumor cells during tumor metastasis16,17,18,19,20,21,22. Several changes in cellular phenotypes are considered to define EMT programs. Among these, three changes are especially important23,24. The first change is morphological, in which a cobblestone-like monolayer of epithelial cells transforms into spindle-shaped mesenchymal cells. The second change is in differentiation markers, in which a decrease is observed in epithelial cell markers (eg, E-cadherin and ZO-1) and an increase is observed in mesenchymal cell markers (eg, vimentin and N-cadherin). Finally, the third change in cell functions is associated with cell invasion and the synthesis of extracellular matrix (ECM).
The data accumulated to date indicate that some anti-PF drugs, such as N-acetylcysteine, have anti-EMT effects25. During the course of PF, activated type II lung epithelial cells undergo EMT and transform into fibroblasts and myofibroblasts, which secrete large amounts of ECM, thereby destroying the lung architecture and accelerating the fibrosis process. A previous report demonstrated that approximately 33% of fibroblasts in the lung tissues of an experimental fibrosis model originated from cells undergoing EMT9. The present study shows that paeoniflorin, a natural compound with substantial anti-PF efficacy, attenuated EMT in the lung tissues of bleomycin-induced mice, as evidenced by its efficient inhibition of the loss of E-cadherin and the expression of FSP-1 and α-SMA. Combined with our previous findings13, we hypothesize that paeoniflorin exerts its anti-PF action by preventing EMT and the consequent synthesis of type I collagen.
To gain insight into the underlying mechanisms by which paeoniflorin inhibits pulmonary EMT, we performed in vitro EMT assays using A549 cells (human type II alveolar epithelial cells). TGF-β1, a typical pro-fibrotic cytokine that has a well-known ability to induce EMT in both rat and human type II alveolar epithelial cells, was used as a stimulator26. It is widely accepted that in the presence of TGF-β1, epithelial cells first transform into mesenchymal cells, then into fibroblasts and, eventually, myofibroblasts. E-cadherin, a typical marker of epithelial cells, is expressed on the surface of epithelial cells. Its expression gradually decreases with EMT progression, during which it can switch to N-cadherin expression, as seen in fibroblasts27. By contrast, vimentin and α-SMA are biomarkers of fibroblasts. Fibroblasts that specifically express α-SMA are called myofibroblasts, and they are the main source of type I collagen during tissue fibrosis. In this study, paeoniflorin was shown to not only dramatically prevent changes in cell morphology and migration in pulmonary epithelial cells but also to inhibit the loss of epithelial cell markers and the acquisition of fibroblast and myofibroblast markers. Paeoniflorin also decreased the secretion of ECM during EMT. A time course assay demonstrated that paeoniflorin mainly affects the early stages of EMT, which includes the processes by which epithelial cells transform into mesenchymal cells.
The loss of E-cadherin is considered a central event in the development of EMT28. The transcription factors responsible for the regulation of E-cadherin expression can be divided into three families: the Snail, ZEB and bHLH families. Of these, the Snail family members (Snail and Slug) are zinc-finger proteins, which function by directly binding to the E-boxes of the E-cadherin promoter to repress the transcription of this gene. The actions of Snail and Slug thus depend on their zinc finger domain and their N-terminal SNAG domain29. When Snail function was blocked using RNA interference, TGF-β was unable to induce EMT in MDCK-Snail cells30. Conversely, the two members of the ZEB family, ZEB1 and ZEB2, have two zinc-finger clusters at each end, which bind to E-boxes and regulate DNA transcription. ZEB proteins can interact with Smad3 to directly repress the expression of E-cadherin in EMT31,32. Furthermore, Twist, a member of the bHLH family, is highly expressed during embryogenesis, tissue fibrosis and tumor invasion33. During the process of EMT, Twist represses the expression of E-cadherin and increases the expression of fibronectin and N-cadherin via a mechanism that is independent of Snail34. In the present study, paeoniflorin was found to dramatically repress the expression of both Snail and Slug mRNA but not of ZEB1, ZEB2 or Twist, implying that the Snail family might be the target of paeoniflorin.
During the EMT processes that are induced by TGF-β, multiple signaling pathways participate in the regulation of Snail expression. They can be roughly classified into Smad-dependent and Smad-independent pathways35. With respect to the Smad-dependent pathways, TGF-β initially phosphorylates its receptor complex, and activated TβRI (TGF-β receptor I) induces the phosphorylation of Smad2 and Smad3. Phosphorylated Smad2 and Smad3, the receptor-regulated Smads (R-Smads), form trimers with their common partner, Smad (Smad4), and then translocate into the nucleus. After entering the nucleus, the complex of Smad2/3-Smad4 associates and cooperates with DNA-binding transcription factors to regulate the expression of Snail36,37. In Smad3-deficient mice, TGF-β fails to induce EMT in renal tubular epithelial cells in unilateral ureteral obstruction-induced renal interstitial fibrosis38. Smad-independent pathways also play an important role in EMT. TGF-β activates the PI3K/Akt and MAP kinase pathways to regulate the expression of transcription factors related to EMT in a manner independent of Smads, resulting in the loss of E-cadherin39. Inhibitors of PI3K, ERK and p38 MAPK have been used to block the development of EMT in HepG2 cells, normal murine mammary gland epithelial cells and pulmonary epithelial cells, respectively40,41,42. In addition, SP600125, a JNK inhibitor, inhibited TGF-β-induced EMT in a PDV cell line43. The present study shows that paeoniflorin has the ability to inhibit the phosphorylation of both Smad2 and Smad3 but it only slightly inhibits the activation of Akt, ERK, JNK and p38 MAPK, suggesting that it down-regulates Snail expression via a Smad-dependent pathway.
Recently, increasing attention has focused on the negative regulation of the Smad-dependent canonical and Smad-independent non-canonical TGF-β pathways. Smad7 is an inhibitory Smad that acts as a negative regulator of signaling by TGF-β superfamily proteins. Data indicate that TGF-β1-induced Smad7 recruits Smad ubiquitin regulatory factor (Smurf) proteins with HECT type E3 ubiquitin ligase activity to suppress the canonical TGF-β pathway via the ubiquitination-mediated proteasomal degradation of ALK5. In addition, the non-canonical TGF-β pathway was blocked by Smad 6 but not Smad 7 through the recruitment of deubiquitinase A20 to TRAF644,45,46. Based on these results, we analyzed the effect of paeoniflorin on the levels of Smad7 and ALK5 in A549 cells. The results showed that paeoniflorin increased the level of Smad7 but decreased the level of ALK5.
In conclusion, in vivo and in vitro assays confirmed that paeoniflorin effectively prevents pulmonary EMT, which provides a reasonable explanation for its inhibitory effects on the excessive synthesis of type I collagen in lung tissues, which consequently results in a reduction in the fibrosis induced by bleomycin in mice. Paeoniflorin mainly acts in the early stages of TGF-β mediated EMT, likely by down-regulating the expression of the transcription factors Snail and Slug via a Smad-dependent canonical pathway involving the up-regulation of Smad7.
Author contribution
Yu JI performed all the in vivo studies, performed the analysis of the collected data and drafted the manuscript; Yan-nong DOU, Qian-wen ZHAO, Ji-zhou ZHANG, Yan YANG, Ting WANG and Yu-feng XIA performed the in vitro studies; Yue DAI and Zhi-feng WEI performed the assays and analyses and drafted the manuscript.
References
Bouros D . Pirfenidone for idiopathic pulmonary fibrosis. Lancet 2011; 377: 1727–9.
Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G . Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2006; 174: 810–6.
Gross TJ, Hunninghake GW . Idiopathic pulmonary fibrosis. N Engl J Med 2001; 345: 517–25.
Polyakova V, Loeffler I, Hein S, Miyagawa S, Piotrowska I, Dammer S, et al. Fibrosis in endstage human heart failure: Severe changes in collagen metabolism and MMP/TIMP profiles. Int J Cardiol 2011; 151: 18–33.
Crouch E . Pathobiology of pulmonary fibrosis. Am J Physiol 1990; 259: L159–84.
Raghu G, Striker LJ, Hudson LD, Striker GE . Extracellular matrix in normal and fibrotic human lungs. Am Rev Respir Dis 1985; 131: 281–9.
Gabbiani G . The biology of the myofibroblast. Kidney Int 1992; 41: 530–2.
Willis BC, duBois RM, Borok Z . Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc 2006; 3: 377–82.
Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 2009; 180: 657–65.
Li JZ, Wu JH, Yu SY, Shao QR, Dong XM . Inhibitory effects of paeoniflorin on lysophosphatidylcholine-induced inflammatory factor production in human umbilical vein endothelial cells. Int J Mol Med 2013; 31: 493–7.
Zhou H, Bian D, Jiao X, Wei Z, Zhang H, Xia Y, et al. Paeoniflorin protects against lipopolysaccharide-induced acute lung injury in mice by alleviating inflammatory cell infiltration and microvascular permeability. Inflamm Res 2011; 60: 981–90.
Zheng YQ, Wei W, Zhu L, Liu JX . Effects and mechanisms of Paeoniflorin, a bioactive glucoside from paeony root, on adjuvant arthritis in rats. Inflamm Res 2007; 56: 182–8.
Ji Y, Wang T, Wei ZF, Lu GX, Jiang SD, Xia YF, et al. Paeoniflorin, the main active constituent of Paeonia lactiflora roots, attenuates bleomycin-induced pulmonary fibrosis in mice by suppressing the synthesis of type I collagen. J Ethnopharmacol 2013; 149: 825–32.
Pirozzi G, Tirino V, Camerlingo R, Franco R, La Rocca A, Liguori E, et al. 2011. Epithelial to mesenchymal transition by TGFβ-1 induction increases stemness characteristics in primary non small cell lung cancer cell line. PLoS One 2011; 6: e 21548.
Smith BN, Odero-Marah VA . The role of Snail in prostate cancer. Cell Adh Migr 2012; 6: 433–41.
Kalluri R, Weinberg RA . The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 199: 1420–8.
Habiel DM, Hogaboam C . Heterogeneity in fibroblast proliferation and survival in idiopathic pulmonary fibrosis. Front Pharmacol 2014; 5: 2.
Günther A, Korfei M, Mahavadi P, von der Beck D, Ruppert C, Markart P . Unravelling the progressive pathophysiology of idiopathic pulmonary fibrosis. Eur Respir Rev 2012; 21: 152–60.
Willis BC, Borok Z . TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 2007; 293: L525–34.
Yang H, Zhan L, Yang T, Wang L, Li C, Zhao J, et al. Ski prevents TGF-β-induced EMT and cell invasion by repressing SMAD-dependent signaling in non-small cell lung cancer. Oncol Rep 2015. doi: 10.3892/or.2015.3961.
Zhang F, Zhang X, Meng J, Zhao Y, Liu X, Liu Y, et al. ING5 inhibits cancer aggressiveness via preventing EMT and is a potential prognostic biomarker for lung cancer. Oncotarget 2015; 6: 16239–52.
Song P, Zheng JX, Xu J, Liu JZ, Wu LY, Liu C . β-Catenin induces A549 alveolar epithelial cell mesenchymal transition during pulmonary fibrosis. Mol Med Rep 2015; 11: 2703–10.
Zeisberg M, Neilson EG . Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009; 119: 1429–37.
Yang J, Weinberg RA . Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008; 14: 818–29.
Felton VM, Borok Z, Willis BC . N-acetylcysteine inhibits alveolar epithelial-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol 2009; 297: L805–12.
Ramos C, Becerril C, Montaño M, García-De-Alba C, Ramírez R, Checa M, et al. FGF-1 reverts epithelial-mesenchymal transition induced by TGF-{beta}1 through MAPK/ERK kinase pathway. Am J Physiol Lung Cell Mol Physiol 2010; 299: L222–31.
Maeda M, Johnson KR, Wheelock MJ . Cadherin switching: essential for behavioral but not morphological changes during an epithelium-to-mesenchyme transition. J Cell Sci 2005; 118: 873–87.
Xu J, Lamouille S, Derynck R . TGF-β-induced epithelial to mesenchymal transition. Cell Res 2009; 19: 156–72.
Nieto MA . The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 2002; 3: 155–66.
Olmeda D, Jordá M, Peinado H, Fabra A, Cano A . Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene 2007; 26: 1862–74.
Peinado H, Olmeda D, Cano A . Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007; 7: 415–28.
Postigo AA, Depp JL, Taylor JJ, Kroll KL . Regulation of Smad signaling through a differential recruitment of co-activators and co-repressors by ZEB proteins. EMBO J 2003; 22: 2453–62.
Massari ME, Murre C . Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol 2000; 20: 429–40.
Vesuna F, van Diest P, Chen JH, Raman V . Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun 2008; 367: 235–41.
Derynck R, Zhang YE . Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003; 425: 577–84.
Flanders KC . Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 2004; 85: 47–64.
Derynck R, Akhurst RJ, Balmain A . TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 2001; 29: 117–29.
Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A . Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 2003; 112: 1486–94.
Moustakas A, Heldin CH . Non-Smad TGF-β signals. J Cell Sci 2005; 118: 3572–84.
Yan W, Fu Y, Tian D, Liao J, Liu M, Wang B, et al. PI3 kinase/Akt signaling mediates epithelial-mesenchymal transition in hypoxic hepatocellular carcinoma cells. Biochem Biophys Res Commun 2009; 382: 631–6.
Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL . Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia 2004; 6: 603–10.
Kolosova I, Nethery D, Kern JA . Role of Smad2/3 and p38 MAP kinase in TGF-β1-induced epithelial-mesenchymal transition of pulmonary epithelial cells. J Cell Physiol 2011; 226: 1248–54.
Santibañez JF . JNK mediates TGF-beta1-induced epithelial mesenchymal transdifferentiation of mouse transformed keratinocytes. FEBS Lett 2006; 580: 5385–91.
Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell 2000; 6: 1365–75.
Jung SM, Lee JH, Park J, Oh YS, Lee SK, Park JS, et al. Smad6 inhibits non-canonical TGF-β1 signalling by recruiting the deubiquitinase A20 to TRAF6. Nat Commun 2013; 4: 2562.
Hneino M, François A, Buard V, Tarlet G, Abderrahmani R, Blirando K, et al. The TGF-β/Smad repressor TG-interacting factor 1 (TGIF1) plays a role in radiation-induced intestinal injury independently of a Smad signaling pathway. PLoS One 2012; 7: e35672.
Acknowledgements
This work was funded by the Innovative Training Plan for Graduate Students of Jiangsu Province (No CXZZ11_0829) and the Priority Academic Program Development of the Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary information is available at the Acta Pharmacologica Sinica's website.
Supplementary information
Supplementary Information Figure S1
The effects of paeoniflorin (Pae) and prednisone (Pred) on the expression levels of FSP-1, α-SMA and E-cadherin on bleomycin-induced fibrosis in mouse lung tissues. (TIF 5337 kb)
Supplementary Information Figure S2
The effects of different concentrations of paeoniflorin (Pae) on TGF-β1-induced epithelial-mesenchymal transition in A549 cells.A549 cells were seeded into plates andincubated with serum-free RPMI 1640 for 2 h. (TIF 2305 kb)
Rights and permissions
About this article
Cite this article
Ji, Y., Dou, Yn., Zhao, Qw. et al. Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol Sin 37, 794–804 (2016). https://doi.org/10.1038/aps.2016.36
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.36
Keywords
This article is cited by
-
Biomaterial-based mechanical regulation facilitates scarless wound healing with functional skin appendage regeneration
Military Medical Research (2024)
-
Scutellarin Alleviates Ovalbumin-Induced Airway Remodeling in Mice and TGF-β-Induced Pro-fibrotic Phenotype in Human Bronchial Epithelial Cells via MAPK and Smad2/3 Signaling Pathways
Inflammation (2024)
-
Protective effects of Qing-Re-Huo-Xue formula on bleomycin-induced pulmonary fibrosis through the p53/IGFBP3 pathway
Chinese Medicine (2023)
-
PRDX1 negatively regulates bleomycin-induced pulmonary fibrosis via inhibiting the epithelial-mesenchymal transition and lung fibroblast proliferation in vitro and in vivo
Cellular & Molecular Biology Letters (2023)
-
Asarinin attenuates bleomycin-induced pulmonary fibrosis by activating PPARγ
Scientific Reports (2023)