Abstract
Chromosomal polymorphism is widespread in the Drosophila genus, with extensive evidence supporting its adaptive character in diverse species. Moreover, inversions are the major contributors to the genus chromosomal evolution. The molecular characterization of a reduced number of polymorphic inversion breakpoints in Drosophila melanogaster and Drosophila subobscura supports that their inversions would have mostly originated through a mechanism that generates duplications —staggered double-strand breaks— and has thus the potential to contribute to their adaptive character. There is also evidence for inversion breakpoint reuse at different time scales. Here, we have characterized the breakpoints of two inversions of D. subobscura —O4 and O8— involved in complex arrangements that are frequent in the warm parts of the species distribution area. The duplications detected at their breakpoints are consistent with their origin through the staggered-break mechanism, which further supports it as the prevalent mechanism in D. subobscura. The comparative analysis of inversions breakpoint regions across the Drosophila genus has revealed several genes affected by multiple disruptions due not only to inversions but also to single-gene transpositions and duplications.
Similar content being viewed by others
Introduction
Chromosomal inversions were first identified in Drosophila melanogaster through their recombination suppressing effect in heterozygotes1. The presence of polytene chromosomes in insects facilitated their subsequent cytological identification in this and other Drosophila species, which opened up the possibility to detect and identify inversions that segregated in natural populations. Dobzhansky pioneered the study of chromosomal polymorphism in natural populations by performing extensive geographical and temporal surveys of inversion frequencies in the Nearctic species D. pseudoobscura (as compiled in Lewontin et al.2). This work prompted similar studies in other Drosophila species such as D. subobscura, D. repleta and D. melanogaster (as summarized in Krimbas and Powell3). These studies provided ample evidence for the adaptive character of chromosomal polymorphism.
In the Drosophila genus, detailed cytological maps based on the banding pattern of polytene chromosomes were built for many species. The availability of these maps allowed locating inversion breakpoints, and it later allowed combining the use of molecular markers and in situ hybridization to molecularly identify and characterize these breakpoints. This is however a laborious endeavor that in the absence of a medium to high quality reference genome sequence of the species under study requires the availability of either other genomic resources such as BAC libraries to narrow down the region under scrutiny, or a good reference genome of a relatively closely related species to use as a guide. The number of polymorphic inversions with breakpoints molecularly characterized is still scanty for Drosophila species4,5,6,7,8,9,10,11,12,13,14. Only in D. melanogaster with one of the best reference genomes, the availability of population genome-wide datasets has allowed to bioinformatically identify the breakpoints of nine polymorphic inversions15,16,17,18,19.
Classical cytological studies of inversion polymorphism in different Drosophila species had revealed that inversion breakpoints are not evenly distributed either among or along chromosomal arms20,21. Moreover, they provided evidence for breakpoint reuse at this short time scale. The comparative analysis of genome sequences across the Drosophila phylogeny also revealed the uneven distribution of the breakpoints of fixed chromosomal rearrangements, providing evidence that some regions had been multiply disrupted22,23. The observed reuse of some breakpoints or breakpoint regions can reflect the breakage-prone character (or fragility) of some genomic regions. However, evolutionary successful rearrangements (both polymorphic and fixed) constitute a subset of those generated. The observed reuse can therefore also reflect the new arrangement effect on fitness.
The characterization of inversion breakpoints in inverted and non-inverted chromosomes allows addressing various important questions concerning among others the possible functional effect of the inversion itself, and the repeated use of breakpoint regions. Inversions can originate through (i) the cut-and-paste mechanism, (ii) unequal recombination between repetitive elements, and (iii) staggered double-strand breaks and their subsequent repair. However, only the latter mechanism generates segmental duplications at the breakpoints of the inverted arrangement24. The characterization of inversion breakpoints also facilitates to later uncover putative targets of selection in the inverted fragment through its effect on nucleotide variation. Our work focusing on five inversions of the E chromosome (Muller’s C element) of D. subobscura that are involved in chromosomal arrangements of the E1+2 complex, has already unveiled that one inversion breakpoint with cytological evidence for having been multiply reused has also been multiply reused molecularly, and that a second breakpoint also considered to be cytologically shared by two inversions is not coincident at the molecular level12,14. Moreover, the molecular structure of these inversions breakpoints and of those of one inversion of the O chromosome (Muller’s E element)11 has also revealed that the staggered-breaks mechanism is probably the most frequently used mechanism to generate inversions in this species. Finally, the comparative analysis of the breakpoint regions of the D. subobscura polymorphic inversions across the Drosophila genus has revealed that some of these regions have been multiply disrupted12,13,14.
Here we have characterized the breakpoints of two additional inversions of the O chromosome of this species —inversions O4 and O8— that, like the five inversions of the E chromosome with breakpoints previously characterized, are involved in generating complex arrangements. Indeed, inversions O4 and O8 are overlapping inversions that occurred sequentially and led from the ancestral and now extinct O3 arrangement to the extant O3+4 and O3+4+8 arrangements (Fig. 1). These arrangements can be present in natural populations also harboring Ost that, like O3+4, originated from the ancestral arrangement, in this case through the O3 inversion (Fig. 1). The frequencies of O3+4 and O3+4+8 in the species ancestral distribution area do not only exhibit a high negative correlation with latitude25 but also a high positive correlation with temperature26, which has led them to be considered warm-adapted arrangements27. The identification and molecular characterization of inversions O4 and O8 breakpoint regions of D. subobscura will further contribute to our understanding of how polymorphic inversions originate and it will also pave the road to identify the genetic basis of their adaptive character. Additionally, the characterization of these regions across the Drosophila genus will shed light on the extent of breakpoint reuse at different time scales.
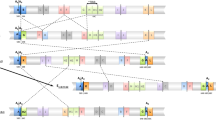
Schematic representation of the O chromosome regions of Drosophila subobscura affected by inversions O3, O4 and O8.
The affected regions are represented in extant gene arrangements Ost, O3+4 and O3+4+8, and in the extinct ancestral O3 chromosomal arrangement of Drosophila subobscura. Horizontal lines represent the different chromosomal arrangements (blue if extant and grey if extinct). Short vertical lines on the Ost arrangement represent the different inversion breakpoints with indication of their location (section) on the Kunze-Mühl and Müller28 map. Double arrows highlight inversion events between arrangements whereas arrows on the left side of the image represent the sequential accumulation of inversions from the ancestral O3 arrangement (grey, the previously characterized inversion O3; blue, inversions O4 and O8). Grey shaded boxes between arrangements indicate the extent of the corresponding inversion. Short colored horizontal lines represent the fragments spanning the breakpoint regions of the inversions. C, centromere; T, telomere.
Results
Identification of breakpoint regions by chromosome walking
We have identified the breakpoints of two inversions —inversions O4 and O8 of the O chromosome— by performing the corresponding chromosomal walks that were guided by the results obtained by in situ hybridization of the different rounds of probes in both non-inverted and inverted chromosomes. Each walk was initiated from a molecular marker that had been previously mapped near the corresponding breakpoint.
O4 inversion breakpoints
The breakpoints of inversion O4 are cytologically located at sections 94D/94E and 98C/98D on the Ost Kunze-Mühl and Müller28 map and therefore between sections 94E/94D and 98C/98D on the ancestral O3 arrangement (Fig. 1). Markers AbdA11 and Obp83a/Obp83b29 that are located at section 94E and 98D, respectively, were used as starting points to identify the inversion breakpoints in non-inverted chromosomes.
For the proximal breakpoint, three rounds of serial in situ hybridizations were needed to design a final probe that putatively would span it (Supplementary text and Supplementary Figure S1). This probe —DO4pOF28— gave a single strong signal at section 94D/94E when hybridized on Ost chromosomes, and two strong signals at sections 94E next to 98C and 94D next to 98D when hybridized on O3+4 chromosomes (Supplementary Figure S2). These results confirmed that this probe spanned the proximal breakpoint of inversion O4 in non-inverted (Ost and O3) chromosomes.
For the distal breakpoint, two rounds of serial in situ hybridizations were needed to design a final probe that would putatively span it (Supplementary text and Supplementary Figure S1). This probe —DO4dOF28—gave a single strong signal at section 98C/98D on Ost chromosomes, and two strong signals at sections 98C next to 94E and 98D next to 94D on O3+4 chromosomes (Supplementary Figure S2). These results confirmed that this probe spanned the distal breakpoint of inversion O4 in non-inverted (Ost and O3) chromosomes.
The identification of both the proximal and distal breakpoints of inversion O4 in Ost chromosomes allowed amplifying the fragments spanning these breakpoints in O3+4 chromosomes with the corresponding combination of oligonucleotides (Fig. 1). Their in situ hybridization on Ost chromosomes gave two signals (Supplementary Figure S2), which confirmed that they included the corresponding breakpoints in O3+4 chromosomes. It should be added that they also gave two signals on O3+4 chromosomes (see next paragraph).
The fragments spanning the breakpoints in Ost and O3+4 chromosomes were completely sequenced and subsequently annotated. The ~5.6-kb long fragment spanning the proximal breakpoint in Ost —DO4pOF28— contains part of the Pxd gene, the CG5225 gene and part of the CG4009 gene, whereas the ~3.8-kb long fragment spanning the distal breakpoint —DO4dOF28— contains part of both the Set8 and Acf genes (Fig. 2). In O3+4, the ~8.7-kb long fragment —DO4pchcu— spanning the proximal breakpoint contains part of the Pxd and Acf genes, and the Set8 gene, whereas the ~5.6-kb long fragment spanning the distal breakpoint —DO4dchcu— contains part of the CG5225 and Acf genes (Fig. 2). The pairwise comparison of fragments spanning the breakpoints in Ost and O3+4 chromosomes allowed delimiting and characterizing the breakpoints. The presence in the O3+4 proximal breakpoint of part of the Acf gene and remnants of the CG5225 gene, and the presence in the distal breakpoint of part of the Pxd gene indicate that these fragments were duplicated during the inversion process. These results would therefore be solely consistent with the O4 inversion having originated through the staggered double-strand break mechanism (Fig. 2). The duplicated fragments would explain the double signal observed at the breakpoint regions in both the Ost and O3+4 arrangements when using as probes the O3+4 breakpoints.
Schematic representation of inversion O4 breakpoint regions in chromosomal arrangements O3 and O3+4.
Colored arrows represent the breakpoint regions annotated as in Fig. 1. Colored and grey boxes represent gene exons and introns, respectively, whereas black dashed lines represent intergenic regions. Red dashed lines along a chromosomal region represent staggered breaks and their limits, whereas dashed lines between chromosomal arrangements indicate the limits and orientation of homologous regions, with numbers indicating their location in the sequenced fragments.
O8 inversion breakpoints
The breakpoints of inversion O8 are cytologically located at sections 90D/91A and 94A/94B (Fig. 1) according to the Kunze-Mühl and Müller28 map. Markers previously located near each breakpoint —DP2_4d at section 91A and trus at section 93D— were used to initiate the corresponding chromosomal walks.
For the proximal breakpoint, four rounds of serial in situ hybridizations were needed to identify the probe that putatively spanned the breakpoint (Supplementary text and Supplementary Figure S3). The DO8pC probe gave a single signal at section 90D/91A on O3+4 (ch cu) chromosomes and two strong signals at the corresponding sections on O3+4+8 (OF40) chromosomes (Supplementary Figure S4). These results confirmed that this probe spanned the proximal breakpoint of inversion O8 in non-inverted (O3+4) chromosomes.
For the distal breakpoint, three rounds of serial in situ hybridizations were needed to design a final probe that putatively spanned it (Supplementary text and Supplementary Figure S3). This probe —DO8dD— gave a single strong signal at section 94B/C on O3+4 (ch cu) chromosomes and two strong signals at the corresponding sections on O3+4+8 (OF40) chromosomes (Supplementary Figure S4). This result confirmed that this probe spanned the distal breakpoint of inversion O8 in non-inverted (O3+4) chromosomes, and that this breakpoint is located at section 94B/C of the Kunze-Mühl and Müller28 map and not at section 94A/B as previously described28.
The identification of both the proximal and distal breakpoints of inversion O8 in O3+4 chromosomes allowed amplifying the fragments spanning these breakpoints in the O3+4+8 chromosomes with the corresponding combination of oligonucleotides (Fig. 1). Their in situ hybridization on O3+4 chromosomes gave two signals (Supplementary Figure S4), a confirmation that they included the corresponding breakpoints in O3+4+8 chromosomes. It should be noted that they also gave two signals on O3+4+8 chromosomes (see next paragraph).
The fragments spanning the breakpoints in O3+4 and O3+4+8 chromosomes were completely sequenced and subsequently annotated. The ~4.8-kb long fragment spanning the proximal breakpoint in O3+4 —DO8pC— contains genes Pli (partial), TfIIA-S and CG12207 (partial), whereas the ~7.2-kb long fragment spanning the distal breakpoint region —DO8dD— contains genes Ald (partial) and Prosβ2R2, and part of the ncRNA CR46041 gene (Fig. 3). In O3+4+8 (OF40) chromosomes, the ~8.0-kb long fragment spanning the proximal breakpoint —DO8p_OF40— contains genes Pli (partial), Prosβ2R2 (with a CMC transposable element insertion) and Ald (partial), whereas the ~8.6-kb long fragment spanning the distal breakpoint —DO8d_OF40— contains genes TfIIA-S and Prosβ2R2, and part of the CR46041 gene (Fig. 3). The pairwise comparison of fragments spanning the breakpoints in O3+4 and O3+4+8 chromosomes allowed delimiting and characterizing the breakpoints. The proximal O8 inversion breakpoint in O3+4 arrangement can be narrowed down to an ~300-bp long stretch. Two small fragments (380- and 128-bp long) flanking this stretch are duplicated at the proximal and distal breakpoints of the O3+4+8 arrangement, respectively. The presence of the Prosβ2R2 gene in the O3+4+8 proximal and distal breakpoints indicates that this gene was duplicated during the repair of the staggered double-strand break that initiated the inversion process.
Schematic representation of inversion O8 breakpoint regions in chromosomal arrangements O3+4 and O3+4+8.
Colored arrows represent the breakpoint regions annotated as in Fig. 1. Colored and grey boxes represent gene exons and introns, respectively, whereas black dashed lines represent intergenic regions. Red dashed lines along a chromosomal region represent staggered breaks and their limits, whereas dashed lines between chromosomal arrangements indicate the limits and orientation of homologous regions, with numbers indicating their location in the sequenced fragments.
Inversions O4 and O8 breakpoint regions in Drosophila
A comparative analysis was performed across the Drosophila phylogeny relative to colinearity breaks near the genes either affected by or flanking the breakpoints of the here studied inversions as well as their immediate neighbors.
Breakpoint regions of the O4 inversion
The comparative analysis of the O4 proximal breakpoint region revealed that the four-genes block present in the Ost (O3) arrangement of D. subobscura —CG4009-CG5225-Pxd-CG8907— predated the diversification of the Drosophila genus given its presence in species of both the Drosophila and Sophophora subgenera (e.g., D. mojavensis and D. subobscura Ost arrangement, respectively; Supplementary Figure S5). However, the presence of gene CG31268 between genes CG4009 and CG5225 in most species of the Sophophora subgenus raises the possibility of a five-genes block — CG4009-CG31268-CG5225-Pxd-CG8907—predating the subgenera split. In the former scenario, gene CG31268 would have been inserted in the Sophophora subgenus ancestor whereas in the second scenario, this gene would have been lost in the ancestor of the Drosophila subgenus. In either case, gene CG31268 would have been subsequently either lost or transposed elsewhere in D. subobscura, and become a pseudogene in D. simulans. The CG5225 gene that would have been lost independently in D. sechellia and D. grimshawi would have undergone an intrachromosomal transposition in the ancestor of D. pseudoobscura and D. persimilis and a microinversion in the ancestor of the melanogaster group. Genes GA31730 in D. pseudoobscura and GL24546 in D. persimilis that exhibit fragments of similarity to gene CG5225 could be either a remnant of the latter gene duplicative transposition or the result of a new gene insertion. Concerning the two genes flanking the proximal O4 inversion breakpoint, a total of at least five intergenic disruptions would have occurred under the first scenario (four under the second scenario) across the Drosophila phylogeny —three (two) at the 5’ upstream region of gene CG5225 and two (two) at its 3’ downstream region— as a result of this gene intrachromosomal transposition and inversion, and the insertion of gene CG31268. Moreover, a disruption affecting genes Pxd and CG5225 would have originated the paracentric inversion that segregates in D. subobscura as part of the O3+4 arrangement.
The comparative analysis of the O4 distal breakpoint region —genes CG42233-Acf-Set8-Afti—revealed that this four-genes block is highly conserved across the Drosophila phylogeny. Indeed, only two disruptions would have occurred in the genus and both would have affected the same intergenic region (between genes Set8 and Acf). Both disruptions can be considered the result of paracentric inversions: inversion O4 originated in D. subobscura, and at least a second inversion predating the melanogaster subgroup diversification.
Breakpoint regions of the O8 inversion
The comparative analysis of the O8 breakpoint regions revealed that they both have a rather complex evolutionary history (Supplementary Figure S6). For the proximal breakpoint that is flanked by genes Pli and TfIIA-S, the presence of the Lsp1β-Pli-TfIIA-S block in species of the Drosophila subgenus as well as in species of the obscura group, constitutes a clear indication of the ancestral character of this 3-genes block. In contrast, the repeated disruption of the upstream region of gene TfIIA-S by at least three paracentric inversions precludes inferring its ancestral neighbor. Moreover, at the downstream region of gene Pli, the Lsp1β gene would have been the subject of two independent duplications plus a microinversion and an interchromosomal transposition to Muller’s B element in the ancestor of the melanogaster subgroup, and it also flanked the breakpoint of a paracentric inversion. Concerning the two genes flanking the proximal O8 inversion breakpoint, a total of at least seven intergenic disruptions would have occurred across the Drosophila phylogeny —three at the 5′ upstream region of gene TfIIA-S, three at the 3′ downstream region of the Pli gene, and one between both genes— as a result of four paracentric inversions, a microinversion and a gene transposition.
The edges of the Prosβ2R2 gene delimit the O8 distal breakpoint. The comparative analysis of this gene and its two neighbors in D. subobscura —Ald-Prosβ2R2-CR31086— revealed that this 3-genes block is only present in species of the obscura group. However, when an extended 5-genes block was considered —CG6154-Ald-Prosβ2R2-CR31086-CG12290—, we could detect that the four genes flanking Prosβ2R2 were present as a block in D. melanogaster and likely also in the remaining nine species as revealed by the three protein coding genes. In the obscura group species, there is a second inverted copy of Prosβ2R2 separated by three coding regions. Moreover, only in species of this group and the melanogaster subgroup is Prosβ2R2 located in Muller’s E element. Its localization in Muller’s A element of D. ananassae and in Muller’s D element of species of the Drosophila subgenus indicates that this gene would have been the subject of at least one interchromosomal transposition. It would have been also affected by at least one intrachromosomal transposition predating the diversification of the obscura group (Supplementary Figure S6), as well as by the paracentric inversion that segregates in D. subobscura as part of the O3+4+8 arrangement and a one-gene duplication and its intrachromosomal transposition in the ancestor of the obscura group.
Discussion
Chromosomal inversion polymorphism is widespread in the Drosophila genus even though it is unevenly distributed across species and also among chromosomal elements of polymorphic species. Drosophila subobscura stands out because its five large acrocentric chromosomes are polymorphic. Muller elements C and E of this species (chromosomes E and O, respectively) are those for which the highest numbers of naturally occurring inversions have been described30. Moreover, both elements present complex systems of inversions—the E1+2 and O3+4 complexes, respectively— resulting from the sequential accumulation of inversions. Upon completing the characterization of the breakpoints of the five inversions of the E chromosome leading from the ancestral Est arrangement to the four most common arrangements of the E1+2 complex —E1+2, E1+2+9, E1+2+9+3, and E1+2+9+12— 12,13,14, we have completed the characterization of the breakpoints of the three inversions leading from the now extinct O3 arrangement to the most common arrangements of the telomere proximal part (segment I) of the O chromosome —Ost11, O3+4 and O3+4+8 (present work)—. Concerning inversions O4 and O8, our results are consistent with their having both originated (like inversions E1, E9, E3, E12 and O3) by the staggered double-strand break mechanism. In the case of inversion O8, the duplicated fragment that is present in inverted orientation at both breakpoints of the O3+4+8 arrangement corresponds to only one breakpoint of the non-inverted O3+4 arrangement, similarly to the five previously mentioned inversions of D. subobscura. In contrast, duplicates corresponding to both breakpoints of non-inverted Ost (or O3) chromosomes are present in inverted orientation at each of the two breakpoints of the O3+4 arrangement. Even though both fragments present at the proximal breakpoint of the latter arrangement included partial genes, only that corresponding to the distal breakpoint of O3 was preserved and could be easily identified as opposed to that corresponding to the O4 proximal breakpoint. Indeed, only parts of the duplicated region could be identified through similarity-based searches in the O4 proximal breakpoint of the O3+4 arrangement. In those cases where the duplicated fragment includes a truncated copy of the gene, the action of purifying selection preserves the functional copy present at the other breakpoint whereas both point and length mutations accumulate through time in the truncated copy due to the relaxation of selection. Also in the case that an inversion originated by ectopic recombination between repetitive elements, the integrity of these elements may be eroded by the accumulation of mutations. Time may thus blur the differential signals left by mechanisms originating inversions.
Concerning the disruptions affecting the breakpoints themselves and also the extended breakpoint regions, our results have revealed (i) one micro-duplication at each the proximal and distal breakpoints of inversion O8 in chromosomal arrangement O3+4+8, and therefore upon the inversion occurrence; (ii) the independent disruption by paracentric inversions of the short intergenic region between genes set8 and Acf in D. subobscura and in the ancestor of the melanogaster subgroup; (iii) the recurrent disruption by paracentric inversions of the short intergenic region upstream of the TfIIA-S gene; and (iv) the involvement of genes CG5225 and Prosβ2R2 in multiple rearrangements in the Drosophila genus that include transpositions, duplications and inversions. It should be noted that genes set8, Acf, TfIIA-S and Prosβ2R2 share some characteristics concerning their expression, as revealed in D. melanogaster. They have at least one Class I insulator near the transcription start site (~50 to 200 nucleotides distance), and Prosβ2R2 also at the end of the transcription unit. Disruptions at the upstream region of any of these genes as well as on both their upstream and downstream regions —as is the case of the detected interchromosomal transpositions of gene Prosβ2R2— would generally not affect their expression given the presence of nearby insulators. There are also diverse indications that the flanking regions of these genes might be breakage-prone. Indeed, the four genes involved in multiple rearrangements are embedded in active chromatin domains with most of them being widely expressed.
Our comparative analysis of gene order changes in D. subobscura and across the Drosophila genus has focused on the genes flanking the breakpoints of the former species polymorphic inversions as well as on their neighboring genes. In D. subobscura, our molecular characterization of the breakpoints of eight polymorphic inversions (five and three of Muller’s C and E element, respectively) has provided ample evidence for multiple disruptions either of the breakpoints themselves12,14 or of the extended breakpoint regions31. Although our analysis has not revealed any general enrichment in low-complexity repetitive sequences flanking inversion breakpoints with molecular evidence for having been reused, it has revealed the presence of two different snoRNAs generating genes next to a gene involved in two sequential inversions sharing a breakpoint31. Our analysis at the long time scale has detected that genes flanking the D. subobscura inversion breakpoints and their neighbors have generally been affected by multiple disruptions. It has also identified some genes that have been individually involved in multiple structural rearrangements and more specifically in at least one intrachromosomal or interchromosomal transposition: genes subito12,31, Lsp1β32 (present work) and Prosβ2R2 (present work). The expression of these genes would not have been affected by the regulatory elements of their diverse neighboring genes through evolutionary time as supported by the detected nearby insulators. This characteristic might render them elusive to the sieving effects of purifying selection when their flanking regions were disrupted, which would increase their probability of being involved in both polymorphic and fixed rearrangements.
In summary, the characterization (in previous11 and present work) of the breakpoints of inversions leading to the most common chromosomal arrangements of the O3+4 complex of D. subobscura has revealed the presence of inverted duplications only at the inverted arrangements breakpoints, which is only consistent with they having all originated by the staggered-breaks mechanism as also did most inversions of the E1+2 complex of this species12,13,14. Moreover, the comparative analysis of the breakpoint regions of inversions involved in the most common arrangements of both complexes across the Drosophila genus further supports that they are prone to participate in evolutionary successful rearrangements as multiple disruptions have been detected at different time scales in these regions. Finally, these analyses have allowed detecting genes involved in multiple and diverse structural rearrangements.
Materials and Methods
Three homokaryotypic strains of D. subobscura were used to molecularly identify the breakpoints of inversions O4 and O8, and to subsequently sequence their breakpoint regions: strains OF28 (Ost), ch cu (O3+4), and OF40 (O3+4+8). The OF strains were obtained through over 13 generations of sibmating from isofemale lines established upon collection in Observatori Fabra (Barcelona, Catalonia, Spain), as reported in Puerma et al.12.
For each inversion, two chromosomal walks were performed to identify its breakpoints using as starting points molecular markers previously mapped in their vicinity. In this procedure, serial sets of probes are in situ hybridized on polytene chromosomes to physically map them. This allows advancing (walking) towards each breakpoint until its final identification. The design of probes was based on colinearity blocks between the D. pseudoobscura and D. melanogaster genomes, as well as on some scaffolds from draft2 of the D. subobscura genome sequence (Barcelona Subobscura Initiative [BSI]) as described in Puerma et al.12. Probes were amplified by PCR using genomic DNA from the ch cu strain, biotin labeled and in situ hybridized on the corresponding strains.
Oligonucleotides for probes amplification were designed directly on D. subobscura sequences. Hybridization signals that were located on the cytological map of D. subobscura28 allowed walking towards each breakpoint and to eventually cross it. All steps of the in situ hybridization procedure were performed as described in Montgomery et al.33. Digital images at a 400 magnification were obtained using a phase contrast Axioskop 2 Zeiss microscope and a Leica DFC290 camera.
Fragments spanning breakpoints were PCR amplified using TaKaRa DNA polymerase (Takara Bio Inc) in both non-inverted and inverted chromosomes, and oligonucleotides anchored at each breakpoint flanking regions. The amplified fragments were sequenced using primer walking whenever necessary. Amplicons were purified with MultiScreen PCR plates (Millipore) prior to their sequencing with the ABI PRISM version 3.2 cycle sequencing kit. Sequencing products separated on an ABI PRISM 3730 sequencer. All sequences were obtained on both strands and assembled using the DNASTAR package34. Sequences newly obtained have been deposited in the EMBL/GenBank Data Libraries under accession numbers LT622817 to LT622824.
Sequence analysis
All breakpoint regions were annotated with genes through their comparison with the D. pseudoobscura genome (FlyBase; http://flybase.org/) using BLAST tools and analyzed with RepeatMasker (http://repeatmasker.org/) to detect transposable elements and other repeated motifs. In order to finely establish each breakpoint and to determine putative duplications resulting from the inversion process, the newly sequenced breakpoint regions of each inversion were compared among them using the Align Sequences Nucleotide BLAST utility at the NCBI webpage.
In order to asses whether the D. subobscura breakpoint regions had also been disrupted at the long time scale, we performed a comparative analysis of these regions based on the first 12 sequenced genomes of the Drosophila genus35. Our analysis focused on the two genes generally affected by a breakpoint (either because the breakpoint laid in an intergenic region or because the staggered break affected both genes) and in one case on one gene, as it was the only gene affected by the staggered breaks. In order to infer the type of structural change that had led to a particular disruption, we included in the analysis the minimum number of neighboring genes needed for that purpose.
Different FlyBase utilities were used to identify the orthologs of the genes present in the extended breakpoint regions of the D. subobscura inversions in Drosophila species other than D. melanogaster. Orthologs were initially identified using GBrowse searches. However, the lack of annotation or misannotation of some genes in one or more of the eleven species, as well as a different relative orientation of the genes included in a particular block in some species, led us to manually curate those regions using BLAST tools (including blastn and tblastn).
The phylogenetic analysis of the breakpoint extended regions generally allowed us to infer the type of structural change that had been fixed and in which branch it had originated and become fixed. The disruption of two neighboring genes (or blocks of genes) was considered to be the result of a paracentric inversion when the distance between these genes in the genome with the derived arrangement was very large. Genes within a gene block that in some of the analyzed species are located in a different Muller element than its neighbors are considered to have undergone an interchromosomal transposition. In those cases where the putative transposition affects a single species, the gene location should be considered with some caution as it could also be due to miss-assembly of genome sequences generated by Next Generation Sequencing (NGS).
Class I insulators that act as gene regulatory boundaries allow the independent spatial and temporal expression of adjacent genes. This class of insulators as well as an active chromatin state exhibit a positive association with synteny breaks in the Drosophila genus36. Both the function of Class I insulators and the detected associations motivated including these characteristics in our comparative analysis. We therefore retrieved information from FlyBase on Class I insulators and chromatin state at the gene regions included in our analysis.
Additional Information
How to cite this article: Puerma, E. et al. Multiple and diverse structural changes affect the breakpoint regions of polymorphic inversions across the Drosophila genus. Sci. Rep. 6, 36248; doi: 10.1038/srep36248 (2016).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Sturtevant, A. H. A case of rearrangement of genes in Drosophila. Proc. Natl. Acad. Sci. USA 7, 235–237 (1921).
Lewontin, R. C., Moore, J. A., Provine, W. B. & Wallace, B. Dobzhansky’s Genetics of Natural Populations I–XLIII (Columbia University Press, 1981).
Krimbas, C. B. & Powell, J. R. Drosophila inversion polymorphism (CRC Press, 1992).
Wesley, C. S. & Eanes, W. F. Isolation and analysis of the breakpoint sequences of chromosome inversion In(3L)Payne in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 91, 3132–3136 (1994).
Andolfatto, P., Wall, J. D. & Kreitman, M. Unusual haplotype structure at the proximal breakpoint of In(2L)t in a natural population of Drosophila melanogaster. Genetics 153, 1297–1311 (1999).
Cáceres, M., Ranz, J. M., Barbadilla, A., Long, M. & Ruiz, A. Generation of a widespread Drosophila inversion by a transposable element. Science 285, 415–418 (1999).
Casals, F., Cáceres, M. & Ruiz, A. The Foldback-like transposon Galileo is involved in the generation of two different natural chromosomal inversions of Drosophila buzzatii. Mol. Biol. Evol. 20, 674–685 (2003).
Matzkin, L. M., Merritt, T. J. S., Zhu, C.-T. & Eanes, W. F. The structure and population genetics of the breakpoints associated with the cosmopolitan chromosomal inversion In(3R)Payne in Drosophila melanogaster. Genetics 170, 1143–1152 (2005).
Richards, S. et al. Comparative genome sequencing of Drosophila pseudoobscura: Chromosomal, gene, and cis-element evolution. Genome Res. 15, 1–18 (2005).
Delprat, A., Negre, B., Puig, M. & Ruiz, A. The transposon Galileo generates natural chromosomal inversions in Drosophila by ectopic recombination. PLoS One 4, e7883; doi: 10.1371/journal.pone.0007883 (2009).
Papaceit, M., Segarra, C. & Aguadé, M. Structure and population genetics of the breakpoints of a polymorphic inversion in Drosophila subobscura. Evolution (N.Y.) 67, 66–79 (2013).
Puerma, E. et al. Characterization of the breakpoints of a polymorphic inversion complex detects strict and broad breakpoint reuse at the molecular level. Mol. Biol. Evol. 31, 2331–2341 (2014).
Puerma, E., Orengo, D. J. & Aguadé, M. The origin of chromosomal inversions as a source of segmental duplications in the Sophophora subgenus of Drosophila. Sci. Rep. 6, 30715; doi: 10.1038/srep30715 (2016).
Orengo, D. J., Puerma, E., Papaceit, M., Segarra, C. & Aguadé, M. A molecular perspective on a complex polymorphic inversion system with cytological evidence of multiply reused breakpoints. Heredity (Edinb.) 114, 610–618 (2015).
Corbett-Detig, R. B. & Hartl, D. L. Population genomics of inversion polymorphisms in Drosophila melanogaster. PLoS Genet. 8, e1003056; 10.1371/journal.pgen.1003056 (2012).
Lack, J. B. et al. The Drosophila genome nexus: A population genomic resource of 623 Drosophila melanogaster genomes, including 197 from a single ancestral range population. Genetics 199, 1229–1241 (2015).
Corbett-Detig, R. B., Cardeno, C. & Langley, C. H. Sequence-based detection and breakpoint assembly of polymorphic inversions. Genetics 192, 131–137 (2012).
Langley, C. H. et al. Genomic variation in natural populations of Drosophila melanogaster. Genetics 192, 533–598 (2012).
Pool, J. E. et al. Population genomics of sub-Saharan Drosophila melanogaster: african diversity and non-African admixture. PLoS Genet. 8, e1003080; 10.1371/journal.pgen.1003080 (2012).
Bernstein, N. & Goldschmidt, E. Chromosome breakage in structural heterozygotes. Am. Nat. 95, 53–56 (1961).
Sperlich, D. & Pfriem, P. In The genetics and biology of Drosophila (eds Ashburner, M., Carson, H. L. & Thompson, J. N. ) 3e, 257–309 (Academic Press London, 1986).
Bhutkar, A., Russo, S. M., Smith, T. F. & Gelbart, W. M. Genome-scale analysis of positionally relocated genes. Genome Res. 17, 1880–1887 (2007).
Von Grotthuss, M., Ashburner, M. & Ranz, J. M. Fragile regions and not functional constraints predominate in shaping gene organization in the genus Drosophila. Genome Res. 20, 1084–1096 (2010).
Ranz, J. M. et al. Principles of genome evolution in the Drosophila melanogaster species group. PLoS Biol. 5, e152, doi: 10.1371/journal.pbio.0050152 (2007).
Prevosti, A. et al. Colonization of America by Drosophila subobscura: Experiment in natural populations that supports the adaptive role of chromosomal-inversion polymorphism. Proc. Natl. Acad. Sci. USA 85, 5597–5600 (1988).
Menozzi, P. & Krimbas, C. B. The inversion polymorphism of Drosophila subobscura revisited: synthetic maps of gene arrangement frequencies and their interpretation. J. Evol. Biol. 5, 625–641 (1992).
Rego, C. et al. Clinal patterns of chromosomal inversion polymorphisms in Drosophila subobscura are partly associated with thermal preferences and heat stress resistance. Evolution (N.Y.) 64, 385–397 (2010).
Kunze-Mühl, E. & Müller, E. Weitere Untersuchungen uber die chromosomale Struktur und die natürlichen Strukturtypen von Drosophila subobscura Coll. Chromosoma 9, 559–570 (1958).
Sánchez-Gracia, A. & Rozas, J. Molecular population genetics of the OBP83 genomic region in Drosophila subobscura and D. guanche: contrasting the effects of natural selection and gene arrangement expansion in the patterns of nucleotide variation. Heredity (Edinb.) 106, 191–201 (2011).
Krimbas, C. B. In Drosophila Inversion Polymorphism (eds Krimbas, C. B. & Powell, J. R. ) 127–220 (CRC Press, 1992).
Puerma, E., Orengo, D. J. & Aguadé, M. Evidence for a gene involved in multiple and diverse rearrangements in the Drosophila genus. Mol. Biol. Evol. 31, 2998–3001 (2014).
González, J., Casals, F. & Ruiz, A. Duplicative and conservative transpositions of Larval serum protein 1 genes in the genus Drosophila. Genetics 168, 253–264 (2004).
Montgomery, E., Charlesworth, B. & Langley, C. H. A test for the role of natural selection in the stabilization of transposable element copy number in a population of Drosophila melanogaster. Genet. Res. 49, 31–41 (1987).
Burland, T. G. DNASTAR’s Lasergene sequence analysis software. Methods Mol. Biol. 132, 71–91 (2000).
Consortium, D. 12 G. Evolution of genes and genomes on the Drosophila phylogeny. Nature 450, 203–218 (2007).
Nègre, N. et al. A comprehensive map of insulator elements for the Drosophila genome. PLoS Genet. 6, e1000814; 10.1371/journal.pgen.1000814 (2010).
Acknowledgements
We thank David Salguero for his excellent technical assistance. We also thank Servei de Genòmica, Serveis Cientifico-Tècnics, Universitat de Barcelona, for automated sequencing facilities. This paper was prepared with full knowledge and support of the Barcelona Subobscura Initiative (BSI). This work was supported by grants BFU2012-35168 and BFU2015-63732 from Ministerio de Economía y Competitividad, Spain, and 2014SGR-1055 from Comissió Interdepartamental de Recerca i Innovació Tecnològica, Generalitat de Catalunya, Spain to MA.
Author information
Authors and Affiliations
Contributions
E.P., D.J.O. and M.A. conceived the study; E.P. and D.J.O. conducted the experiments; E.P., D.J.O. and M.A. analyzed the results. All authors reviewed the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Puerma, E., Orengo, D. & Aguadé, M. Multiple and diverse structural changes affect the breakpoint regions of polymorphic inversions across the Drosophila genus. Sci Rep 6, 36248 (2016). https://doi.org/10.1038/srep36248
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep36248
This article is cited by
-
Basal hsp70 expression levels do not explain adaptive variation of the warm- and cold-climate O3 + 4 + 7 and OST gene arrangements of Drosophila subobscura
BMC Evolutionary Biology (2020)
-
Long-read based assembly and synteny analysis of a reference Drosophila subobscura genome reveals signatures of structural evolution driven by inversions recombination-suppression effects
BMC Genomics (2019)
-
The molecular genealogy of sequential overlapping inversions implies both homologous chromosomes of a heterokaryotype in an inversion origin
Scientific Reports (2019)
-
The molecular characterization of fixed inversions breakpoints unveils the ancestral character of the Drosophila guanche chromosomal arrangements
Scientific Reports (2019)
-
Inversion evolutionary rates might limit the experimental identification of inversion breakpoints in non-model species
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.