Abstract
Aromatic amines containing an aminobenzene or an aniline moiety comprise versatile natural and artificial compounds including bioactive molecules and resources for advanced materials. However, a bio-production platform has not been implemented. Here we constructed a bacterial platform for para-substituted aminobenzene relatives of aromatic amines via enzymes in an alternate shikimate pathway predicted in a Pseudomonad bacterium. Optimization of the metabolic pathway in Escherichia coli cells converted biomass glucose to 4-aminophenylalanine with high efficiency (4.4 g L−1 in fed-batch cultivation). We designed and produced artificial pathways that mimicked the fungal Ehrlich pathway in E. coli and converted 4-aminophenylalanine into 4-aminophenylethanol and 4-aminophenylacetate at 90% molar yields. Combining these conversion systems or fungal phenylalanine decarboxylases, the 4-aminophenylalanine-producing platform fermented glucose to 4-aminophenylethanol, 4-aminophenylacetate and 4-phenylethylamine. This original bacterial platform for producing artificial aromatic amines highlights their potential as heteroatoms containing bio-based materials that can replace those derived from petroleum.
Similar content being viewed by others
Introduction
The production of bulk materials and fuels from renewable resources is prerequisite for constructing sustainable low-carbon societies that can overcome global environmental issues and limited fossil fuel resources. Aromatic amines that are characterized by an amino-substituted benzene (aniline) moiety (referred to hereinafter as AA) serve as resources from which to develop dyes, rubbers, plastics and conductive polymers1,2 and they are important in a broad range of industries. Most living organisms produce the AA, 4-aminobenzoic acid, as a biosynthetic precursor of folate, which is an essential cofactor that is also a dietary supplement3. Some AA are intermediates of antibacterial chloramphenicol, pristinamycin4,5 and other drugs and developing a repertoire of AA is important from a pharmaceutical standpoint. Due to such substantial demand, various commercial AA have been synthesized by petroleum chemistry, whereas none has been derived from biomass, which limits the molecular design of practical bio-derived products based on AA.
Aromatic amines are significant in the production of advanced polymer materials including functional and/or high-performance plastics. The amine group and the aromatic moiety of AA induce nucleophilic reactivity and excellent thermomechanical performance, respectively6. Aromatic amines are polycondensed with carbonyl compounds to generate aromatic polyamides, polyimides, polyazoles, polyurea and polyazomethines. When polycondensed with aromatic acids, AA generate super-engineering plastics with extremely high thermomechanical properties7,8. These include poly(p-phenylene terephthalamide (KevlarTM) and poly(4,4′-oxydiphenylene pyromellitimide) (KaptonTM) that serve as thermostable materials in fabric for body armor and other flame-retardant materials, fiber-reinforced plastics for electronic devices, vehicle bodies and anti-pressure cylinders7,8,9. The applications of super-engineered plastics are diversifying and this is increasing the annual global production of AA-derived plastics to around 100,000 tons. Global production of aromatic polyamides accounts for several hundreds of millions of US dollars, which indicates the size of the contribution of AA to both the economy and society.
The production of aromatic polyamides and polyimides requires aromatic carboxylic acids and/or AA as building blocks. Whereas aromatic carboxylic acids (such as terephthalic acid) have been derived from biomass10,11, AA have not and this has precluded the development of fully bio-oriented aromatic polyamides and polyimides. Our recent microbial production of the non-natural AA, 4-aminocinnamic acid (4ACA), from 4-aminophenylalanine (4APhe), which is an intermediate of the chloramphenicol and pristinamycin biosynthesis pathway4,5, followed by synthesis of ultra-high-performance polyimide is the exception12. Not only 4ACA, but also other AA derived from biomass would serve as innovative monomers for synthesizing bio-AA plastics and their environmental impact should be enormous, considering that they would replace polyamides and polyimides derived in bulk from petroleum.
Only a few natural pathways of AA biosynthesis have been documented. This causes considerable difficulty when trying to custom-design pathways to synthesize various AA. The 4ACA production system ferments 4APhe via antibiotic-synthesizing enzymes produced by recombinant Escherichia coli13 and then a bacterium producing phenylalanine ammonia lyase converts 4APhe to 4ACA (Fig. 1)12. This study identified a novel 4APhe synthetic gene cluster from Pseudomonas fluorescens, which in accordance with the optimization of host E. coli metabolism enables 35-fold more 4APhe production than that generated in a previous study that used antibiotic-synthesizing enzymes from Streptomyces bacteria (0.13 g L−1)13. Recombinant E. coli strains producing sets of phenylalanine-catabolic enzymes fermented glucose to a series of artificial 4-amino-substituted AA including 4-aminophenylacetate (4APAA), 4-aminophenylacetaldehyde, 4-aminophenylethanol (4APE) and 4-aminophenylethylamine (4APEA) (Fig. 1). Bio-aromatic polyamide and polyimides can be developed from all of these compounds. The platform for synthesizing bio-derived AA constructed in the present study increases the potential for producing a range of bio-based AA materials for bio-polymers and other applications.
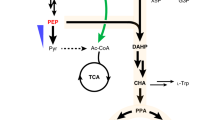
Artificial pathways producing aromatic amines.
AAAD, aromatic amino acid decarboxylase; ADH, aldehyde dehydrogenase; ALDH, alcohol dehydrogenase; AT, aminotransferase; PAL, phenylalanine ammonia lyase; PapA, 4-amino-4-deoxychorismate synthase; PapB, 4-amino-4-deoxychorismate mutase; PapC, 4-amino-4-deoxyprephenate dehydrogenase; PDC, phenylpyruvate decarboxylase. Host cell genes encoded aroG4fbr, feedback resistant isozyme of DAHP synthase; pps, PEP synthase; tktA, transketolase.
Results
Novel P. fluorescens papABC genes optimize 4APhe production
We and another group expressed the Streptomyces venezuelae and S. pristinaespiralis genes encoding 4-amino-4-deoxychorismate synthase (PapA), 4-amino-4-deoxychorismate mutase (PapB) and 4-amino-4-deoxyprephenate dehydrogenase (PapC) in recombinant E. coli and synthesized 4APhe13. These enzymes convert cellular chorismate to 4-aminophenylpyruvate4,5, which endogenous aminotransferases in E. coli subsequently convert to 4APhe13. Homology searches identified similar amino acid sequences to S. venezuelae PapA, PapB and PapC in predicted proteins of P. fluorescens SBW25 (Fig. 2a) encoded by PFLU_1771 (pfpapA), PFLU_1772 (pfpapB) and PFLU_1770 (pfpapC) and their entire amino acid sequences have 44%, 28% and 34% similarity, respectively (Fig. 2a, Supplementary Fig. 1). These genes are clustered in the P. fluorescens SBW25 genome like their Streptomyces counterparts4,5, where they might constitute an operon. We constructed an artificial operon comprising the T7lac gene promoter followed by pfpapB, pfpapA and pfpapC in that order (plasmid 1 in Fig. 2a) and introduced it into E. coli NST37(DE3)/ΔpheLA. The strain lacks a pheLA locus in the leader peptide for pheA expression (pheL) and chorismate mutase (pheA) that competes with the substrate chorismate with PapA. The strain harboring the operon generated 0.43 g L−1 4APhe in minimal medium with glucose whereas all strains expressing only pfpapA, pfpapAB (Fig. 2b), pfpapBC, or the set of pfpapA and pfpapC (Supplementary Fig. 2) did not. These results indicated that pfpapABC participates in 4APhe synthesis.
Identification and use of pfpapABC for 4APhe production.
(a) Comparison of putative pfpapABC gene cluster of P. fluorescens SBW25 and Streptomyces papABC (upper panel). Plasmid organization for pfpapBAC expression (lower panel). (b) HPLC chromatograms of culture supernatant of E. coli after culture in 100 mL of fermentation medium in 500-mL flasks at 30 °C for 30 h. Numbers of introduced plasmids (panel a) are shown on traces. (c) Concentration of 4APhe in culture supernatant of E. coli harboring combinations of plasmids numbered as in (panel a). Error bars indicate standard deviation (n = 3).
We analyzed the ability of a series of pfpapABC expression plasmids to produce 4APhe. The appropriate genes were introduced into pET-duet1, pRSF-duet1 and pCDF-duet1, which were maintained as ~40, 20~40 and >100 copies/cell, respectively14 and expressed under the T7lac promoter (Fig. 2a). The strains harboring sets of pET-pfpapA and pCDF-pfpapBC and pCDF-pfpapA and pET-pfpapBC (plasmids 3, 8 and 5, 6 in Fig. 2c, respectively) produced the most 4APhe, up to 0.53 g L−1. We selected NST37(DE3)/ΔpheLA harboring pET-pfpapA and pCDF-pfpapBC (NDP strain), which produced 4APhe and optimized its 4APhe production.
Metabolic engineering E. coli for efficient AA production
We overexpressed the transketolase gene (tktA) under the control of the T7lac gene promoter in the NDP strain and found that it produced 1.2-fold more 4APhe than the parent strain (Fig. 3a), which is consistent with reports that its overexpression increases the availability of erythrose 4-phosphate (E4P) for 3-deoxy-D-arabinoheptulosonate 7-phosphate (DAHP) synthase, the rate-limiting enzyme of chorismate production and hence cellular chorismate (Fig. 1)15,16 that is a substrate of the PapABC pathway. The enzyme phosphoenolpyruvate (PEP) synthase is used to synthesize cellular PEP that is another substrate of shikimate pathway besides E4P. Overexpression of the PEP synthase gene (pps) and both tktA and pps did not increase 4APhe production (Fig. 3a), indicating that pps decreased 4APhe production under our culture conditions. The PEP-dependent carbohydrate: phosphotransferase system (PTS) is a carbohydrate uptake process in E. coli where it is the major consumer of intracellular PEP17. Aiming to eliminate PEP consumption by PTS during cellular glucose uptake, we disrupted the ptsHI-crr genes that encode the cytoplasmic components of the system. Introducing pET-pfpapA/pCDF-pfpapBC into the gene disruptant resulted in lower 4APhe production compared with the corresponding non-disrupted strain (Supplementary Table 3), probably due to a decreased rate of glucose uptake.
Metabolic engineering of E. coli to optimize 4APhe production.
(a) 4APhe production by E. coli NDP expressing pfpapABC cultured in 100 mL of fermentation medium in 500-mL flasks at 30 °C for 30 h. Ectopically supplemented genes are shown below. Data are means of three experiments. Error bars indicate standard deviation. (b) Fed-batch cultivation of E. coli NDPG strain expressing pfpapABC and aroG4fbr. Fermentation medium contained more tryptone and yeast extract (5 and 2.5 g L−1, respectively). Glucose was fed to maintain concentration <1 g L−1. Error bars indicate standard deviation (n = 3).
We overexpressed aroG that encodes DAHP synthase to provide more chorismate for 4APhe production (Fig. 1). We used the aroG4fbr gene that encodes a feedback inhibition-resistant (fbr) isozyme of DAHP synthase18. The NDPG strain overexpressing aroG4fbr in the NDP strain produced 1.8-fold more 4APhe than the NDP strain in flask cultures (Fig. 3a) and this became more obvious when nitrogen sources in the culture medium were increased (Supplementary Table 4). Therefore, the following tests included bacteria cultured under these conditions. Overexpressing tktA and pps did not increase 4APhe production by the NDPG strain (Supplementary Table 4), whereas it increased the production of phenylalanine by a strain sharing the same aroGfbr background15. These findings indicate that the amount of DAHP synthase limits bacterial 4APhe production under these conditions. We fed-batch cultured NDPG in a jar fermenter and optimized the conditions for 4APhe production. The glucose concentration decreased to <1 g L−1 after 16 h of batch culture and was maintained at this level thereafter to avoid repressing T7lac gene promoter activity19 and the metabolic overflow of glucose to acetate that inhibits E. coli fermentation20. Fed batch-cultured NDPG generated 4.4 g L−1 4APhe with a production yield of 17% (vs. glucose) (Fig. 3b), which represents 62% of the theoretical yield of phenylalanine by E. coli21.
Production of 4APE and 4APAA
The fungal Ehrlich pathway deaminates phenylalanine to phenylpyruvate, which phenylpyruvate decarboxylase (PDC) then converts to phenylacetaldehyde. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) reduce and oxidize phenylacetaldehyde to 2-phenylethanol and to phenylacetic acid, respectively22. Artificial pathways mimicking the Ehrlich pathway were designed to produce 4APE and 4APAA (Fig. 1) via pathway enzymes, although a 4-amino-substituted AA has never been assessed as an Ehrlich pathway intermediate. We constructed recombinant E. coli BL21 Star (DE3) producing ARO10 encoding PDC from the fungus S. cerevisiae22 and examined the bioconversion of 4APhe in resting cells. The reaction consumed 1.8 g L1 (10 mM) of 4APhe to generate 1.2 g L−1 of 4APE with a 90% molar yield (Table 1), implying that PDC efficiently produced 4-aminophenylacetaldehyde, which endogenous E. coli ADH reduced to 4APE. The resting E. coli BL21 Star (DE3) cells producing both ARO10 and ALDH encoding ALD322 converted 1.8 g L−1 of 4APhe to 0.9 g L−1 of 4APAA with a 63% molar yield (Table 1). The strain produced 0.4 g L−1 4APE as a byproduct, indicating that the dehydrogenation of 4-aminophenylacetaldehyde limits 4APAA production. Replacing ALD3 with E. coli padA23 and S. cerevisiae ALD222 in the bacterium expressing ARO10 and ALD3 increased the yields of 4APAA to 85% and 90%, respectively (Table 1). We also combined PDC, Pichia pastoris PpARO1024 or Aspergillus oryzae ppdA25 with ALD3, but they produced less 4APAA than those expressing ARO10 and ALD3 (Table 1). These results indicated that the heterologous expression of ARO10 alone and of the ARO10 and ALD2 set converted 4APhe to 4APE and APAA the most efficiently. Figure 4a,b shows the time-dependent bioconversion of 4APhe (3.6 g L−1) in the optimized systems that produced 2.8 g L−1 4APE and 2.7 g L−1 4APAA.
Production of 4APE and 4APAA.
(a,b) Bioconversion of 4APhe to 4APE and 4APAA by E. coli BL21 Star (DE3) harboring either pRSF-aro10 (a) or pRSF-aro10ald2 (b). After incubation with 0.1 mM IPTG, cells were washed twice and incubated in reaction buffer containing 3.6 g L−1 4APhe. Error bars indicate standard deviation (n = 3). (c, d) HPLC chromatogram of culture supernatant of NDPG harboring pRSF-aro10 (c) and pRSF-aro10ald2 (d). Cells were shaken in fermentation medium containing 5 g L−1 tryptone, 2.5 g L−1 yeast extract and 10 g L−1 glucose in flasks at 30 °C, induced with 0.1 mM IPTG and cultured for 36 h.
We expressed ARO10 or both ARO10 and ALD2 in the NDPG strain expressing pfpapABC. The resulting NDPGA and NDPGAA strains cultured in fermentation medium produced 4APE and both 4APAA and 4APE, respectively. Production increased linearly until reaching a maximum of 0.24 g L−1 4APE (Fig. 4c), 0.12 g L−1 4APAA and 0.19 g L−1 4APE (Fig. 4d) at 36 h. These results showed that the cultures fermented glucose to 4APE and 4APAA. Both strains accumulated less fermentation products than the intermediate, 4APhe (0.4–1.2 g L−1) (Fig. 4c,d). Further optimization of growth parameters or genetic modification could hasten conversion of the intermediate and produce more 4APE and 4APAA. Fermentation by the NDPGAA generated 4APE as a byproduct (Fig. 4d), while bioconversion by E. coli BL21 Star (DE3) expressing ARO10 and ALD3 resulted in little 4APE production (Fig. 4b), indicating metabolic differences between the strains. These results demonstrated that the new bacterial platform can efficiently produce biomass-derived AA.
Production of 4APEA
We examined the reaction of aromatic L-amino acid decarboxylase, which decarboxylates phenylalanine to phenylethylamine and carbon dioxide, against 4APhe (Fig. 1). We constructed recombinant E. coli BL21 Star (DE3) overexpressing A. oryzae aadA25 and either of the Solanum lycopersicum LeAADC1A and LeAADC1B26 genes, both of which encode aromatic L-amino acid decarboxylases. Incubating these cells with 1.8 g L−1 (10 mM) 4APhe efficiently converted 4APhe to 4APEA with 65% to 75% molar yield (vs. 4APhe) (Fig. 5a). The strain expressing either LeAADC1A or LeAADC1B produced 4APEA within 4 h. We introduced these genes into the NDPG strain and cultured it in fermentation medium containing 1% glucose. After 42 h, 1.3-fold more 4APEA was accumulated by the strain expressing LeAADC1A than LeAADC1B (Fig. 5b), indicating that both strains harboring LeAADC1A converted glucose biomass to 4APEA more efficiently. Fed-batch cultures of the strain expressing LeAADC1A in a jar fermenter generated 1.8 g L−1 4APEA after 44 h (Fig. 5c). The production yield (vs. glucose) of 4APEA was 5.6%, which was below its theoretical yield (23%) calculated from that for phenylalanine (27.5%)21.
Production of 4APEA.
(a) Bioconversion of 4APhe to APEA by E. coli harboring pRSF-aadA, pRSF-leaadc1A and pRSF-leaadc1B. Cells grown in flasks of LB medium containing 0.1 mM IPTG at 30 °C were incubated with 1.8 g L−1 4APhe. Error bars indicate standard deviation (n = 3). (b) Fermentation of 4APEA by NDPG harboring pRSF-leaadc1A or pRSF-leaadc1B. Cells were grown at 30 °C in flasks of fermentation medium containing 5 g L−1 tryptone and 2.5 g L−1 yeast extract and 0.1 mM IPTG. Error bars indicate standard deviation (n = 3). (c) Fed-batch culture of NDPG harboring pRSF-leaadc1A in jar fermenters containing 0.5 L of fermentation medium containing 5 g L−1 tryptone and 2.5 g L−1 yeast extract at 30 °C. Adding ammonium maintained pH at 7.0. Glucose (0.8 g L−1 h−1) was fed at 16 h after starting culture. Error bars indicate standard deviation (n = 3).
Discussion
The aim of constructing microbial platforms to produce various groups of chemicals is to develop specific pharmaceuticals and biomass-derived materials that can substitute for petroleum chemicals. Most current targets for petroleum-based chemicals comprise aliphatic molecules, whereas only aromatic amino acids are fermented using glucose biomass as a carbon donor. Glucose is bio-converted into shikimate pathway intermediates27 and deoxy-scyllo-inosose28, both of which are chemically derivatized to aromatic compounds such as catechol and its related compounds. Bio-derived terephthalic acid has recently been synthesized using biomass-derived isobutanol or 5-hydroxymethylfurfral11. These aromatic compounds were generated by combining microbial, with chemical reactions. However, we explored biological production systems that do not require chemical processes to produce artificial aromatic chemicals, especially those with a substituted nitrogen atom. This is the first bacterial platform for the direct fermentative production of a series of AA and represents a promising environmentally-friendly alternative for synthesizing AA building blocks that are applicable to numerous technologies.
This study integrated metabolic engineering strategies to produce AA. Firstly, established strategies for high-level fermentation of phenylalanine as well as perturbed metabolic flow of chorismate to phenylalanine improved chorismate synthesis by E. coli. Secondly, introducing exogenous papABC genes integrated the intrinsic E. coli pathway for chorismate synthesis with the pathway generating aminophenylpyruvate from chorismate and fermented glucose to 4APhe (Fig. 1). We found that papABC is indispensable for AA production and that the P. fluorescens papABC identified herein and which originated from a proteobacterium similar to E. coli optimized production efficiency. Thirdly, we searched bacteria, fungi and plants for enzymes that produce shikimate pathway derivatives and used them to produce 4APEA, 4APE and 4APAA from a sugar biomass. The production rates are sufficient to generate industrial quantities of pharmaceutical intermediates. Fed-batch fermentation produced optimal amounts of 4APEA (1.8 g L−1), which will be improved by applying traditional strategies such as continuous culture and genetically engineering host E. coli metabolism and 4APEA generated in this manner will have potential for bio-material production after a purification process is established.
The platform produced biomass-derived aromatics containing an aniline structure with a substitution at the para-position via a C2 chain linked to a functional (amine or carbonyl) group. Such structures have unique molecular properties; 4APEA is a diamine connected to rigid aromatic (C6) moiety and flexible C2 chain. This and structurally similar bipartite diamines are polymerized with both aliphatic and aromatic dicarboxylic acids to generate thermostable polyamides with a considerably high glass-transition temperature29. Polyamide generated from 4APEA and isophthalic acid has high thermal stability (Td10 = 360 °C)30. Polycondensation with long flexible aliphatic acids generates 4APEA with liquid crystallinity. Furthermore, 4APAA is a C6:C2 compound and an aromatic amino acid. The related 4-aminobenzoic acid (C6:C1) is homopolymerized to form poly(p-benzamide) with high thermomechanical properties but its poor solubility and the absence of a melting temperature causes difficulties with processing them into plastics. Copolymerizing 4-aminobenzoic acid with 4APAA increases flexibility through the addition of alkyl chains connected to the aromatic rings of poly(p-benzamide)31. This molecular design improves processability into heat-resistant filaments, fibers and films31. Thus, the present findings should impact the development of novel bio-based aromatic materials.
We developed an AA production platform based on a shikimate pathway variant for synthesizing 4APhe (Fig. 1). Although only a small variety of compounds are derived from this pathway in nature, the platform produced the artificial 4-amino-substituted compounds 4APAA, 4APE and 4APAE in combination with the enzymes for synthesizing their phenylalanine derivatives. Natural derivatives of phenylalanine and its synthetic intermediates are more diverse than 4APhe-related compounds and include cinnamic acid, cinnamyl alcohol, cinnamaldehyde, homogentisic acid, phenyllactic acid, mandelic acid, styrene, benzoic acid and other aromatic compounds32. We use phenylalanine-deaminating phenylalanine ammonia lyase (Fig. 1) in the bioconversion of 4APhe to 4ACA11. Other enzymes that biosynthesize phenylalanine derivatives are potential catalysts in the generation of artificial AA, but their synthesis awaits investigation. The constructed platform for AA synthesis has the potential to produce bio-derived non-natural AA.
Methods
Bacterial strains and reagents
Supplementary Table 1 lists the strains used in this study. Escherichia coli NST37 (ATCC31882) was lysogenized using λDE3 Lysogenization kits (Novagen, Madison, WI, USA) to generate NST37 (DE3). We obtained 4APhe from Sigma Aldrich (St. Louis, MO, USA) and 4ACA, 4APE and 4APEA from Tokyo Chemical Industry (Tokyo, Japan). Plasmids were constructed using PrimeSTAR HS DNA polymerase and restriction enzymes (Takara Bio Inc., Shiga, Japan).
Constructing plasmids for 4APhe production
Supplementary Table 2 lists the primers used in this study. Artificially synthesized, codon-optimized pfpapA, pfpapB and pfpapC (GenScript, NJ, USA) (accession numbers; KU199222, KU199223 and KU199224) were cloned into pUC57 to generate pUC-pfpapA, pUC-pfpapB and pUC-pfpapC, respectively. DNA fragments for pfpapA, pfpapB and pfpapC were amplified by PCR using these plasmids and the respective primers sets, PFAF/PFAR, PFBF/PFBR and PFCF/PFCR and then fused by PCR using the primers PFBF and PFCR to generate pfpapBAC fragments. These fragments were digested with NdeI and XhoI and cloned into pET22b to generate pET-pfpapBAC. Fragments of pfpapBA were amplified by PCR using PFBF and PFAR2 primers and pET-pfpapBAC, digested with NdeI and XhoI and cloned into pET22b to generate pET-pfpapBA. We digested pUC-pfpapA with NdeI and XhoI and then purified pfpapA fragments were cloned into pET-duet1, pCDF-duet1 and pRSF-duet-1 (Novagen) that were digested with the same enzymes to generate pET-pfpapA, pCDF-pfpapA and pRSF-pfpapA. We digested pUC-pfpapB with NcoI and NotI and inserted them into the same vectors to generate pET-pfpapB, pCDF-pfpapB and pRSF-papB. We digested pUC-pfpapC with NdeI and XhoI and cloned pfpapC fragments into these plasmids to generate pET-pfpapBC, pCDF-pfpapBC and pRSF-papBC.
Genetic engineering of intrinsic E. coli metabolic pathways
The synthesized DNA fragment aroG4fbr 18 was cloned into pACYC184 that was digested with EcoRV and HindIII and treated with the Klenow fragment to create pACYC-aroG4. The tktA and pps genes were amplified by PCR using E. coli MG1655 total DNA and primers, digested with NcoI and BamHI and NdeI and XhoI and cloned into pRSFduet-1 to generate pRSF-tktA and pRSF-pps, respectively. The pps gene was digested with NdeI and XhoI and also cloned into pRSF-tktA to generate pRSF-tktApps.
Knock-out cassettes generated by PCR using the primers DpheLAF and DpheLAR and the Red/ET recombination system (Gene Bridges, Heidelberg, Germany) replaced the genomic pheLA locus of E. coli NST37(DE3) with the kanamycin resistance gene (Kmr). The FLP/FRT recombination technique (Gene Bridges) deleted Kmr from the strain and generated E. coli NST37(DE3)/ΔpheLA. Diagnostic PCR proceeded using the primers C1 and C2. Likewise, the ptsHI-crr genes of NST37(DE3)/ΔpheLA were knocked-out using the cassette-generated primer pairs DptsHI-crrF and DptsHI-crrR to generate NST37(DE3)/ΔpheLA/ΔptsHI-crr. Primer pairs C3 and C4 was used for diagnostic PCR.
Constructing plasmids for production of other AA
The ARO10 and ALD3 genes were amplified by PCR using total DNA from S. cerevisiae and appropriate primers. The ARO10 fragment was digested with NcoI and BamHI and cloned into pRSFduet-1 to generate pRSF-aro10. The ALD3 fragment was digested with NdeI and XhoI and cloned into pRSFduet-1 and pRSF-aro10 to generate pRSF-ald3 and pRSF-aro10ald3, respectively. The S. cerevisiae ALD2 and the E. coli padA genes were amplified using the appropriate primers, digested with NdeI and XhoI and cloned into pRSF-aro10 to generate pRSF-aro10ald2 and pRSF-aro10padA. The PpARO10 (GenBank accession number, CCA40086.1) gene was amplified by PCR using Pichia pastoris total DNA and primers, digested with SacI and NotI and then cloned into pRSF-ald3 to generate pRSF-pparo10ald3. The A. oryzae ppdA was amplified using primers, digested with NcoI and HindIII and cloned into pRSFduet-1 to generate pRSF-ppdA. The NdeI and XhoI fragment of ALD3 was cloned into pRSF-ppdA to generate pRSF-ppdAald3.
Solanum lycopersicum LeAADC1A and LeAADC1B were amplified using its cDNA and the appropriate primers. The A. oryzae aadA gene was amplified using pET-aadA and the appropriate primers. These DNA fragments were digested with BamHI and HindIII and cloned into pRSFduet-1 to generate pRSF-leaadc1a, pRSF-leaadc1b and pRSF-aadA.
Fermentation of 4APhe, 4APAA, 4APE and 4APEA
Cells were grown in the fermentation medium comprising 10 g glucose, 2 g tryptone, 1 g yeast extract, 6 g Na2HPO4, 3 g KH2PO4, 0.5 g NaCl, 2 g NH4Cl, 0.5 g MgSO4 7H2O, 15 mg CaCl2, 20 mg tyrosine, 20 mg tryptophan, 20 mg phenylalanine, 50 mg thiamine-HCl and 1 mL of trace element solution/L33. The plasmids were maintained by adding 100 mg L−1 sodium ampicillin, 40 mg L−1 kanamycin sulfate and 35 mg L−1 chloramphenicol. Recombinant E. coli generated from NST37(DE3)/ΔpheLA was pre-cultured by rotary shaking at 300 rpm overnight in test tubes containing 5 mL of LB medium at 28 °C and inoculated into 100 mL of fresh M9 medium in 500-mL conical flasks at 1:100 dilution. When the cells reached an optical density (OD) of 0.6 at 600 nm and 30 °C, 0.1 mM isopropyl-β-d-thiogalactoside (IPTG) was added. After 20–24 h of cultivation the glucose concentration reached <2 g L−1 and then 2 mL of 500 g L−1 glucose was added and the cells were further cultured in the flasks for 48 h. Fed-batch cultures were agitated at 30 °C and 550 rpm in a 1.0-L BMJ-01 fermenter (Biott, Tokyo, Japan) containing 0.5 L of fermentation medium supplemented with 5 g L−1 tryptone, 2.5 g L−1 yeast extract, 10 g L−1 (NH4)2SO4 and 10 g L−1 glucose. The culture was aerated at 0.6 L min−1. When the OD reached 0.6 at 600 nm, 0.1 mM IPTG was added. Peristaltic pumps fed the cultures with 500 g L−1 of glucose and 0.1 mM IPTG when the glucose concentration dipped below 1.5 g L−1. The pH was monitored using an electrode and maintained between 7.0 and 7.1 by adding 10% NH4OH.
Bioconversion by resting cells
Escherichia coli BL21 Star (DE3) (Invitrogen, Carlsbad, CA, USA) harboring expression plasmids was rotary shaken at 300 rpm and 30 °C overnight in test tubes containing 3 mL of LB medium and inoculated into 500-mL conical flasks containing 100 mL LB medium at a 1:100 dilution. When the cells reached an OD of 0.6 at 600 nm and 30 °C, 0.1 mM IPTG was added. Cells harboring pRSF-aro10, pRSF-aro10ald3, pRSF-aro10ald2 or pRSF-aro10padA were further incubated at 30 °C for 6 h. The cells were collected by centrifugation at 5,000 × g for 10 min and washed with DCD buffer (100 mM KH2PO4 (pH 7.5), 1 mM MgSO4, 0.5 mM thiamine chloride). Cells harboring pRSF-leaadc1a, pRSF-leaadc1b or pRSF-aadA were further incubated at 16 °C for 12 h with 0.5 mM pyridoxal 5-phosphate, washed with AAD buffer (100 mM potassium phosphate, pH 7.5 and 0.5 mM pyridoxal 5-phosphate). The washed cells were incubated in 10 mL of DCD or AAD buffer containing the indicated amounts of 4APhe at 28 °C and shaken at 300 rpm for the indicated periods.
Determination of metabolites
We determined yields of 4APhe, 4APA, 4APE and 4APEA by high-performance liquid chromatography (HPLC) using a 1200 infinity series (Agilent Technologies, Palo Alto, CA, USA) equipped with a 250 × 4.6-mm Purospher Star RP-18 end-capped column with a particle size of 5 μm (Millipore-Merck, Billerica, MA, USA). The initial mobile phase was solvent A:solvent B = 98:2 (solvent A, 20 mM potassium phosphate (pH 7.0); solvent B, methanol) and maintained for 7 min. The concentration of solvent B was increased to 50% for 5 min and then maintained at that ratio for another 5 min. The flow rate was 0.8 mL min−1 and absorption at 210 nm was monitored. Glucose concentrations were determined using glucose-CII test kit (Wako, Tokyo, Japan).
Additional Information
How to cite this article: Masuo, S. et al. Bacterial fermentation platform for producing artificial aromatic amines. Sci. Rep. 6, 25764; doi: 10.1038/srep25764 (2016).
References
Lawrence, A. S. Amines: synthesis, properties and applications. Cambridge University Press (2006).
Sousa, A. C., Martins, L. O. & Robalo, M. P. Laccase-catalysed homocoupling of primary aromatic amines towards the biosynthesis of dyes. Adv. Synth. Catal. 355, 2908–2917 (2013).
Krishnaswamy, K. & Madhavan Nair, K. Importance of folate in human nutrition. Br. J. Nutr. 85, S115–124 (2001).
He, J., Magarvey, N., Piraee, M. & Vining, L. C. The gene cluster for chloramphenicol biosynthesis in Streptomyces venezuelae ISP5230 includes novel shikimate pathway homologues and a monomodular non-ribosomal peptide synthetase gene. Microbiol. 147, 2817–2829 (2001).
Blanc, V. et al. Identification and analysis of genes from Streptomyces pristinaespiralis encoding enzymes involved in the biosynthesis of the 4-dimethylamino-L-phenylalanine precursor of pristinamycin I. Mol. Microbiol. 23, 191–202 (1997).
Fink, J. K. High Performance Polymers. William Andrew Inc. (2008).
Liaw, D. J. et al. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 37, 907–974 (2012).
García, J. M., García, F. C., Serna, F. & de la Peña, J. L. High-performance aromatic polyamides. Prog. Polym. Sci. 35, 623–686 (2010).
Sapurina, I. The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. Polym. Int. 57, 1295–1325 (2008).
Pacheco, J. J. & Davis, M. E. Synthesis of terephthalic acid via Diels-Alder reactions with ethylene and oxidized variants of 5-hydroxymethylfurfural. Proc. Natl Acad. Sci. USA 111, 8363–8367 (2014).
Lin, Z., Nikolakis, V. & Ierapetritou, M. Alternative approaches for p-xylene production from starch: techno-economic analysis. Ind. Eng. Chem. Res. 53, 10688–10699 (2014).
Suvannasara, P. et al. Biobased polyimides from 4-aminocinnamic acid photodimer. Macromolecules 47, 1586–1593 (2014).
Mehl, R. A. et al. Generation of a bacterium with a 21 amino acid genetic code. J. Am. Chem. Soc. 125, 935–939 (2003).
Tolia, N. H. & Joshua-Tor, L. Strategies for protein coexpression in Escherichia coli. Nat. Methods 3, 55–64 (2006).
Patnaik, R. & Liao, J. C. Engineering of Escherichia coli central metabolism for aromatic metabolite production with near theoretical yield. Appl. Environ. Microbiol. 60, 3903–3908 (1994).
Sprenger, G. A. From scratch to value: engineering Escherichia coli wild type cells to the production of L-phenylalanine and other fine chemicals derived from chorismate. Appl. Microbiol. Biotechnol. 75, 739–749 (2007).
Rodriguez, A. et al. Engineering Escherichia coli to overproduce aromatic amino acids and derived compounds. Microb. Cell Fact. 13, 126 (2014).
Kikuchi, Y., Tsujimoto, K. & Kurahashi, O. Mutational analysis of the feedback sites of phenylalanine-sensitive 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase of Escherichia coli. Appl. Environ. Microbiol. 63, 761–762 (1997).
Grossman, T. H., Kawasaki, E. S., Punreddy, S. R. & Osburne, M. S. Spontaneous cAMP-dependent derepression of gene expression in stationary phase plays a role in recombinant expression instability. Gene 209, 95–103 (1998).
Gerigk, M. et al. Process control for enhanced L-phenylalanine production using different recombinant Escherichia coli strains. Biotechnol. Bioeng. 80, 746–754 (2002).
Förberg, C., Eliaeson, T. & Häggström, L. Correlation of theoretical and experimental yields of phenylalanine from non-growing cells of a rec Escherichia coli strain. J. Biotechnol. 7, 319–331 (1988).
Kim, B., Cho, B. R. & Hahn, J. S. Metabolic engineering of Saccharomyces cerevisiae for the production of 2-phenylethanol via Ehrlich pathway. Biotechnol. Bioeng. 111, 115–124 (2014).
Ferrandez, A., Prieto, M. A., Garcia, J. L. & Diaz, E. Molecular characterization of PadA, a phenylacetaldehyde dehydrogenase from Escherichia coli. FEBS Lett. 406, 23–27 (1997).
Küberl, A. et al. High-quality genome sequence of Pichia pastoris CBS7435. J. Biotechnol. 154, 312–320 (2011).
Masuo, S., Osada, L., Zhou, S., Fujita, T. & Takaya, N. Aspergillus oryzae pathways that convert phenylalanine into the flavor volatile 2-phenylethanol. Fungal Genet. Biol. 77, 22–30 (2015).
Tieman, D. et al. Tomato aromatic amino acid decarboxylases participate in synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc. Natl Acad. Sci. USA 103, 8287–8292 (2006).
Li, W., Xie, D. & Frost, J. W. Benzene-free synthesis of catechol: interfacing microbial and chemical catalysis. J. Am. Chem. Soc. 127, 2874–2882 (2005).
Kogure, T., Wakisaka, N., Takaku, H. & Takagi, M. Efficient production of 2-deoxy-scyllo-inosose from d-glucose by metabolically engineered recombinant Escherichia coli. J. Biotechnol. 129, 502–509 (2007).
Lee, L. T. C. High-transition-temperature polyamides based on 2(2-aminophenyl)1,1-dimethylethylamine. J. Polym. Sci. 16, 2025–2038 (1978).
Ueda, M., Morishima, M. & Kakuta, M. Synthesis of sequential polyamide by direct polycondensation II. Polym. J. 23, 1511–1517 (1991).
Wang, H. H. & Lin, M. F. Modification of nylon-6 with wholly rigid poly(m-phenylene isophthalamide). J. Appl. Polym. Sci. 43, 259–269 (1991).
Thompson, B., Machas, M. & Nielsen, D. R. Creating pathways towards aromatic building blocks and fine chemicals. Curr. Opin. Biotechnol. 36, 1–7 (2015).
Hutner, S. H. Anaerobic and aerobic growth of purple bacteria (athiorhodaceae) in chemically defined Media. Microbiol. 4, 286–293 (1950).
Acknowledgements
We thank Norma Foster for critical reading of the manuscript. We thank Drs. Ariizumi, T. and Ezura, H. (University of Tsukuba) for providing S. lycopersicum cDNA. This work was supported by Advanced Low Carbon Technology Research and Development Program (5100270) and CREST from the Japan Science and Technology Agency.
Author information
Authors and Affiliations
Contributions
S.M. and N.T. designed research and analyzed data. S.M., T.K. and N.T. wrote the paper. S.M. and S.Z. performed the experiments.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Masuo, S., Zhou, S., Kaneko, T. et al. Bacterial fermentation platform for producing artificial aromatic amines. Sci Rep 6, 25764 (2016). https://doi.org/10.1038/srep25764
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep25764
This article is cited by
-
Advances in engineering microbial biosynthesis of aromatic compounds and related compounds
Bioresources and Bioprocessing (2021)
-
Metabolic engineering of microorganisms for production of aromatic compounds
Microbial Cell Factories (2019)
-
Metabolic engineering and transcriptomic analysis of Saccharomyces cerevisiae producing p-coumaric acid from xylose
Microbial Cell Factories (2019)
-
Split intein-mediated selection of cells containing two plasmids using a single antibiotic
Nature Communications (2019)
-
Production of p-amino-l-phenylalanine (l-PAPA) from glycerol by metabolic grafting of Escherichia coli
Microbial Cell Factories (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.